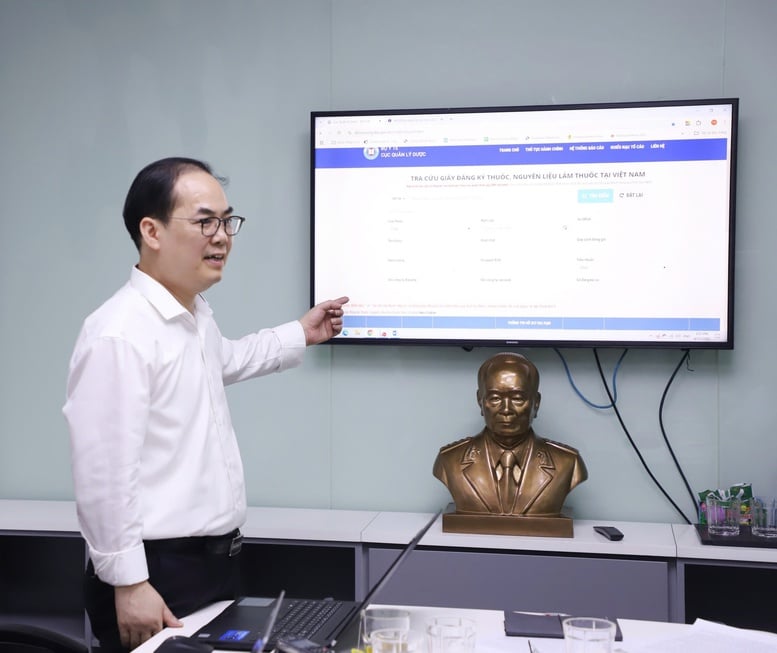
Dr. Ta Manh Hung shows people and businesses how to identify genuine drugs that have been registered for circulation at https://dichvucong.dav.gov.vn/congbothuoc - Photo: VGP/TH
On the afternoon of April 17, Dr. Ta Manh Hung, Deputy Director of the Department of Drug Administration, Ministry of Health, had an interview with the press about the fight against counterfeit drugs related to the Thanh Hoa Provincial Police recently dismantling a large-scale fake pharmaceutical production and trading ring nationwide, arresting 14 subjects for the crime of producing and trading counterfeit disease prevention and treatment drugs.
Counterfeit drugs distributed under the guise of smuggled goods and hand-carried goods
Sir, Thanh Hoa Provincial Police have just discovered a large-scale case of manufacturing and trading counterfeit drugs. It is known that recently, the authorities have also discovered many cases related to counterfeit drugs. Could you please provide more specific information about the above cases?
Dr. Ta Manh Hung: In 2023 and 2024, some localities such as Thanh Hoa, Ha Nam, and Hanoi reported the discovery of a number of fake batches of Tetracycline, Clorocid, Pharcoter, and Neo-Codion. The Ministry of Health promptly directed the Departments of Health to proactively coordinate with Steering Committee 389, the Police, Market Management, etc. to focus on combating, detecting, and promptly handling them in accordance with legal regulations.
In early 2025, due to the complicated situation, the Ministry of Health proactively invited the police and 3 localities with many reports related to fake drugs in the year, namely Thanh Hoa, Ha Tinh, and Ha Nam, to work together.
In this case, according to the report of the Thanh Hoa Provincial Department of Health, the drugs were not officially distributed. The subjects rented a warehouse in a remote area, produced the drugs in a closed system, then sent the subjects to market and sell them online.
The subjects involved in the production and distribution of fake drugs to the market are closely connected and colluded with each other from the production stage to the stage of finding distribution channels and reaching consumers. The subjects operate through social networking sites such as Zalo, Facebook, etc. under the guise of being pharmacists selling drugs for pharmaceutical companies.
These products are advertised as having antibiotics from genuine companies "smuggled" from contractors or sold out of regions, and cannot be invoiced, so they are sold cheaper than genuine products from the companies.
For "fake" products of foreign origin, the subjects introduce the products as "hand-carried" goods without accompanying invoices and documents, to gain the trust of buyers.
In addition, subjects often buy real drugs mixed with fake drugs produced and sold on the market to deal with inspections by authorities.
Once they have a certain number of customers, the subjects only sell self-produced fake drugs, most of the customers are aimed at the group of pharmacists who sell drugs freely at the drug markets.
According to the current preliminary report, all counterfeit drugs were not granted a circulation registration certificate. The subjects counterfeited the labels of drugs that had been licensed for circulation.
Specifically, among the counterfeit drugs recently discovered by Thanh Hoa Provincial Police, there are 4 types of counterfeit modern medicines (Tetracycline, Clorocid, Pharcoter, Neo-Codion).
17 other types of counterfeit products, suspected to be oriental medicines. The products have labels stating their intended use as medicines. The subjects invented the names of the medicines and the production locations to print on the labels, deceiving consumers.
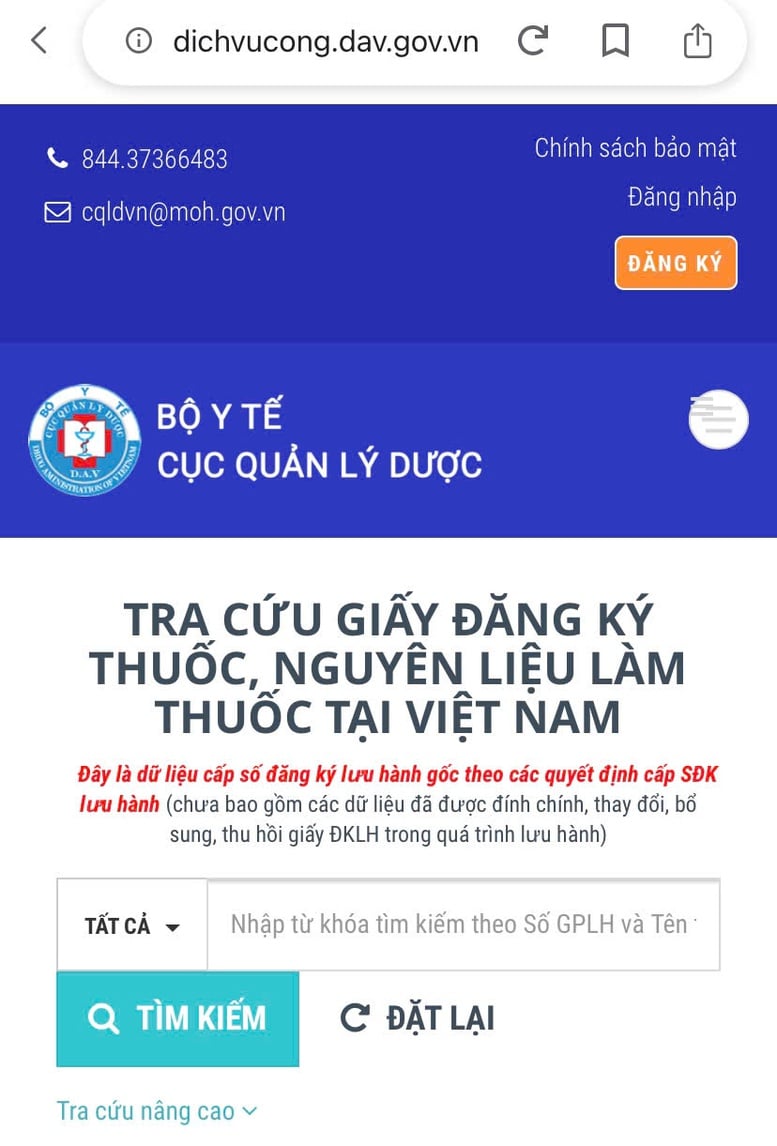
Public service portal to look up drug circulation registration certificates - Photo: VGP/HM
Are there loopholes in pharmaceutical management?
With the above incident, are there any loopholes in the management work, sir? How is the management of drugs and prevention of counterfeit drugs currently being implemented?
Dr. Ta Manh Hung: Every year, the Central Institute for Drug Testing and the Ho Chi Minh City Institute for Drug Testing base on the actual situation of drug quality violations, market developments, and drug quality monitoring and supervision on the market to plan sampling and implement testing during the year.
These plans focus on groups of drugs at high risk of counterfeiting, drugs in high demand, and drugs with a high potential for quality changes during circulation on the market. The plans are built on very detailed and specific sets of principles and assessments.
Then, the units and testing centers of the provinces and cities coordinate with testing institutes to carry out sampling and testing.
Each year, the system of units takes about 38,000-40,000 samples of drugs circulating on the market to test and monitor quality.
According to the report, among the sampled drugs, the rate of substandard drugs was less than 1%, and counterfeit drugs was less than 0.1%.
The Law on Pharmacy also stipulates that drugs are divided into many categories, including vaccines and biological products. For vaccines and biological products, 100% of batches must be inspected, evaluated, and tested to meet quality standards before being released from the factory.
For pharmaceuticals, chemical drugs, experimental drugs, traditional drugs... control is carried out based on risk, sampling for evaluation, monitoring, and quality control.
In addition, competent authorities also focus on implementing the principles of risk management, probability, and sampling for drugs with high risk of counterfeiting and poor quality when in circulation.
People can easily look up and identify real medicine.
After this incident , as a management agency, what solutions will the Drug Administration have to coordinate more effectively in preventing counterfeit drugs, sir?
Dr. Ta Manh Hung: The fight against counterfeit drugs is a key task of the Ministry of Health, but it requires the coordination of many ministries and sectors in inspecting and supervising the fight against counterfeit drugs and drugs of unknown origin to be effective.
In this particular case and some previous cases, we found that counterfeit drugs are mainly sold online and through small distribution channels.
According to regulations, drugs must be granted a circulation registration certificate before being circulated on the market. This information is publicly available on the Public Service Portal at: https://dichvucong.dav.gov.vn/congbothuoc.
People and businesses can look up information to know whether a drug product has been licensed for circulation or not. This lookup is very easy.
For example, the 17 types of counterfeit products recently discovered by Thanh Hoa Provincial Police have no information on the Public Service Portal at the above address. The 4 types of counterfeit pharmaceutical drugs have the same name as the registered drugs but different addresses, batch numbers, etc.
All drugs are licensed for safety and effectiveness before being circulated. During the circulation process, the Ministry of Health, the Ministry of Health Inspectorate, the Department of Health, and the Department Inspectorate all conduct planned inspections and checks and conduct surprise inspections when there are complaints for timely handling.
I would like to emphasize that if the units follow current regulations, buy and sell drugs to the right subjects, at the right establishments with business conditions and have invoices and documents, then there will certainly be no counterfeit drugs or drugs of unknown origin entering the market.
The Ministry of Health determined that in the coming time, there will need to be closer coordination between the Departments of Health and local authorities and local police agencies to make the work of preventing counterfeit drugs more effective.
Thank you!
Thuy Ha (performed)
Source: https://baochinhphu.vn/thuoc-gia-va-kiem-soat-lo-hong-trong-cong-tac-quan-ly-duoc-102250417204910269.htm



![[Photo] Prime Minister Pham Minh Chinh receives delegation from the US-China Economic and Security Review Commission of the US Congress](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/7/ff6eff0ccbbd4b1796724cb05110feb0)























































































Comment (0)