Photo: Science Daily
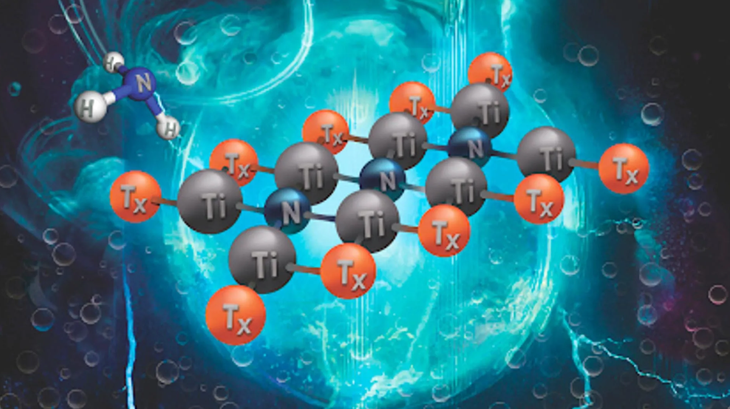
This new material was developed by researchers at Texas A&M University. With a structure that can be tuned at the atomic level, MXene is considered a potential catalyst to replace the expensive materials today.
MXenes are a class of transition metal carbides and nitrides that can catalyze electrochemical reactions to synthesize ammonia from air. The unique chemical properties of MXenes allow scientists to tailor their composition and structure to optimize performance in renewable energy applications.
The work, carried out by professors Abdoulaye Djire and Perla Balbuena and a team of graduate students, was published in the Journal of the American Chemical Society.
The group focuses on expanding the traditional understanding of the behavior of transition metal catalytic materials, toward the possibility of producing chemicals and fuels from abundant resources.
The performance of MXene depends on how the nitrogen atoms interact in the crystal lattice, which determines its vibrational properties and catalytic ability. The ability to fine-tune these properties makes MXene suitable for a wide range of energy applications and is considered a promising alternative to conventional electrocatalyst materials.
The team also used computational simulations to understand how the solvent interacts with the MXene surface, and applied Raman spectroscopy to analyze the structure and reactivity of nitrogen in the crystal lattice.
These results help to elucidate the electrocatalytic mechanism and open up the prospect of controlling ammonia synthesis at the atomic level.
Source: https://tuoitre.vn/vat-lieu-moi-bien-khong-khi-thanh-nhien-lieu-va-phan-bon-20251107072116677.htm













































































































Comment (0)