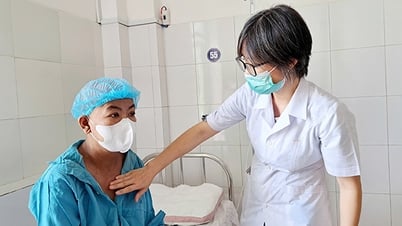
On June 5, the Drug Administration of Vietnam ( Ministry of Health ) announced that it had just issued a document to pharmaceutical production and trading establishments, requesting them to increase production, import, and storage of drugs to prevent and fight COVID-19.
According to Deputy Director of the Drug Administration of Vietnam Le Viet Dung, recently, the COVID-19 epidemic has shown signs of increasing again in some provinces and cities.
In the face of the complicated developments of the COVID-19 epidemic, in order to promptly respond and ensure the supply of drugs for disease prevention and control, the Drug Administration of Vietnam requests pharmaceutical production and trading units to increase the supply of drugs and pharmaceutical ingredients, proactively contact medical examination and treatment facilities to grasp the demand, develop drug production and trading plans to ensure timely supply of drugs to treat Covid-19 and essential drugs to treat COVID-19 patients.
The Drug Administration of Vietnam requires units to focus on symptomatic treatment drugs, respiratory support drugs, emergency resuscitation drugs, etc. according to Decision No. 2671/QD-BYT dated June 26, 2023 of the Ministry of Health promulgating the Guidelines for diagnosis and treatment of COVID-19, ensuring continuous supply, avoiding local drug shortages or supply interruptions.
In cases where drugs are in short supply, the Drug Administration will prioritize the quick resolution of the issuance of circulation registration certificates and import licenses to promptly supply drugs to medical examination and treatment facilities upon request from the units./.
Source: https://www.vietnamplus.vn/bo-y-te-tang-cuong-san-xuat-nhap-khau-thuoc-phong-chong-covid-19-post1042621.vnp


























![[Photo] President Luong Cuong works with Hung Yen and Thai Binh Provincial Party Committees on implementing Resolution of the 11th Central Conference, 13th tenure](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/6/127b735d2761484d81dcee0d7725a25b)


![[Photo] General Secretary To Lam receives Korean Ambassador to Vietnam](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/6/a0765b7543784cbcbfe4755b67d43ab4)























































![[OCOP REVIEW] Tu Duyen Syrup - The essence of herbs from the mountains and forests of Nhu Thanh](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/6/5/58ca32fce4ec44039e444fbfae7e75ec)











Comment (0)