30% of cancer patients with indications for amputation refuse surgery
"At a leading cancer treatment facility, about 30% of cancer patients who are scheduled for amputation refuse surgery, which is a concern for doctors. The reason for refusal is because patients are concerned about physical disabilities after surgery."
That was the opinion shared at the workshop on management and research of 3D printed products in medicine, organized by the Ministry of Health on May 30.

Mr. Nguyen Ngo Quang affirmed that patient safety is paramount when implementing research on 3D printed products in medicine.
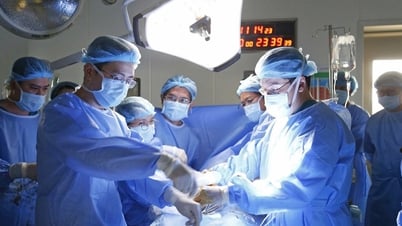
PHOTO: THUY ANH
According to information at the conference, currently, 3D printing in medicine helps create parts that can be replaced after having to be removed during surgery. A few facilities in Vietnam are researching and performing surgery to transplant body parts from 3D printing such as: knee replacement, pelvis, femur, chest reconstruction... 3D printing materials include metal and plastic. Every year, many cancer cases need bone and joint reconstruction to replace after surgery to remove cancerous lesions.
Patient safety comes first
Experts note that the patient's interests must be put first, including assessing the function when replaced; the toxicity of materials to patients when transplanting parts made from 3D printing technology. Patients after transplantation need to be monitored long-term. Cancer patients need lifelong monitoring.
In the draft Law on Medical Devices that is about to be developed, the Ministry of Health needs to have specific regulations and instructions for medical devices that use AI and 3D printing, as a basis for developing documents and implementing instructions, with the standardization of research and application of 3D printing in medicine.
According to Dr. Nguyen Ngo Quang, Director of the Department of Science, Technology and Training (Ministry of Health), 3D printing equipment used in medicine and health care to create personalized medical devices all have risks, so they need to be controlled by management agencies. New, high-risk medical devices need to be clinically tested to assess safety, effectiveness and ease of use. 3D printing materials need to pay special attention to biocompatibility.
Studies must comply with international standards for human studies. The results of the studies demonstrate safety, effectiveness and ease of use.
In particular, Mr. Quang noted, it is necessary to encode products according to patients for long-term management, as well as to monitor health-related issues of patients, if there are unexpected situations, after patients have surgery to replace body parts from 3D printed products.
The Ministry of Health is considering assigning research units and professional associations to develop national standards for standards assigned to production facilities (production conditions, production processes; raw materials, products).
The Department of Infrastructure and Medical Equipment (Ministry of Health) provides guidance on standards, inspection, and licensing. The Department of Science, Technology, and Training is responsible for guiding research.
Source: https://thanhnien.vn/bo-y-te-yeu-cau-an-toan-nguoi-benh-sau-phau-thuat-ghep-xuong-khop-nhan-tao-185250530164529453.htm































































































Comment (0)