The scale could reach nearly 750 million USD.
According to Resolution 57-NQ/TW of the Politburo on the development of science, technology and innovation, by 2030, Vietnam aims to increase investment in research and development (R&D) to 2% of GDP, of which at least 3% of the national budget will be dedicated to this field. This is an important foundation for Vietnam to accelerate its innovation capacity, including the clinical trial industry - a field that is emerging as a new "runway" for medical development and investment attraction.
A report by the Oxford University Clinical Research Unit (OUCRU) indicates that the global clinical trial market is shifting rapidly to Asia, especially Southeast Asia - where pharmaceutical companies are looking for cost-effective, efficient, and patient-diverse research locations.
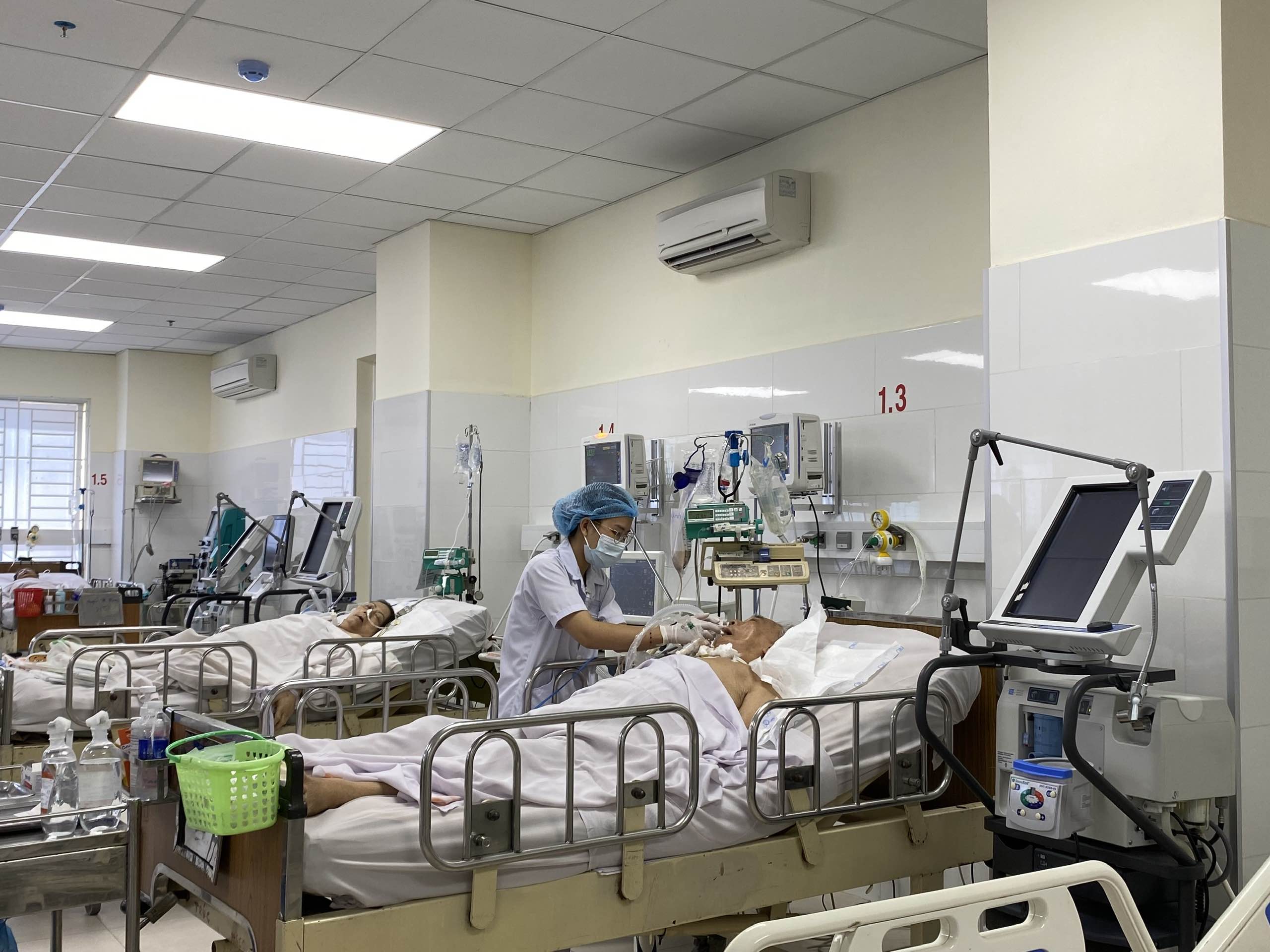 |
Clinical trials give patients access to cutting-edge treatments at low or no cost. |
Grasping this trend, Vietnam is facing a golden opportunity. According to KPMG estimates, the clinical trial market in Vietnam could reach a size of 749.5 million USD by 2029, with a compound annual growth rate (CAGR) of 24.3%, even reaching 88.6% if effectively promoted. Along with that, thousands of high-quality jobs will be created and the ability to access modern treatment methods for domestic patients.
Specialist Doctor II Phan Tan Thuan, Head of the Clinical Trial Unit (CRU) at Ho Chi Minh City Oncology Hospital emphasized: “Cancer is the leading burden in Vietnam and globally. Clinical trials are not only research, but also a chance to live for many patients, who are treated with new drugs, which can cure even stage 4 cancer, without having to pay hundreds of millions or billions of VND.”
In fact, in just 20 years, cancer treatments using immunotherapy and new generation drugs have changed the landscape of treatment. At Ho Chi Minh City Oncology Hospital, the number of clinical trials has increased from a few cases to more than 30 trials running simultaneously, significantly expanding access to new drugs for patients.
In addition to cancer, Vietnam also participates in experimental research in the fields of infectious diseases (Covid-19, tuberculosis, hepatitis, dengue fever, drug resistance), cardiovascular diseases and respiratory diseases.
Many barriers need to be removed
“However, we are still facing many challenges in terms of facilities and professional human resources. We are making efforts to standardize processes, improve capacity and build a research network to meet increasing demands,” Dr. Phan Tan Thuan shared.
Agreeing, Professor Guy Thwaites, Director of the Oxford University Clinical Research Unit, there are three main barriers that are holding back the potential of Vietnam's clinical trials industry.
The first is the lengthy and inflexible procedures. The approval time for a clinical trial in Vietnam can be up to 160 days, the longest in Asia.
Second, there is a shortage of human resources in both quantity and quality. Clinical trials require close coordination between doctors, nurses, study coordinators and data managers. However, Vietnam currently lacks a well-trained team.
Third is the difficulty in financing and incentive mechanisms. A phase 3 trial can cost from 10 to 20 million USD. Meanwhile, many investors reflect that Vietnam lacks tax incentives, research funding or co-financing mechanisms like in Singapore and South Korea.
In addition, disbursement of funds is often delayed due to administrative procedures, affecting testing progress and reducing competitiveness compared to other countries in the region.
Breakthroughs from policy and public-private partnership
To fully exploit this potential, experts say Vietnam needs a short-term, medium-term and long-term strategy. In the immediate future, it is necessary to simplify approval procedures and increase investment incentives to attract resources. At the same time, it is necessary to invest in upgrading infrastructure, developing high-quality human resources and improving R&D capacity at hospitals and research institutes.
In particular, Vietnam needs to promote public-private partnership models and have specialized support policies for clinical trials, inspired by successful countries in the region.
In parallel, it is necessary to expand the network of facilities meeting Good Clinical Practice (GCP) standards, develop clinical trial units (CTUs), and establish a national Center of Excellence (CoE). This will be a connecting point between hospitals, research institutes, universities, and businesses. At the same time, it will be a specialized training center for researchers, contributing to shortening approval times and enhancing global competitiveness.
According to KPMG estimates, if these reforms are effectively implemented, Vietnam could achieve 86 trials with a compound annual growth rate (CAGR) of 24.3% with a market value of 749.5 million USD, the compound annual growth rate in 2029 could reach 88.6%.
This will create thousands of high-quality jobs, enhance Vietnam's global standing in medical research and provide early access to cutting-edge treatments.
Source: https://baodautu.vn/co-hoi-vang-de-phat-trien-thi-truong-thu-nghiem-lam-sang-d299078.html






























































































Comment (0)