
Hong Thai nasal inhaler - Photo: Facebook
On October 29, Nation (Thailand) updated that Thai Herbal Hong Thai Ltd. - the manufacturer of Hong Thai nasal inhaler oil - announced a recall of products from the batch that tested positive for bacterial contamination and pledged to strengthen safety testing procedures.
Previously, the Thai Food and Drug Administration (Thai FDA) discovered that batch 332 of Hong Thai herbal inhaler oil product was contaminated with microorganisms and fungi, warning consumers to be cautious when purchasing and using it.
In response to the above information, Thai Herbal Hong Thai Ltd. announced the recall of 200,000 products from lot 332.
The problem was limited to batch 332 with a production date of December 2024, while other batches were safe and allowed to circulate, said company founder Teerapong Rabueathum.
A representative of Thai Herbal Hong Thai Ltd. confirmed that the information about the suspension of all production and sales of nasal inhaler products is incorrect, because the FDA's announcement only applies to the contaminated product batch.
The company admitted there was an incident related to the detection of microbial contamination exceeding the allowable level in the product, but added that the Thai FDA has yet to announce the specific bacterial strain or explain in detail the health impact.
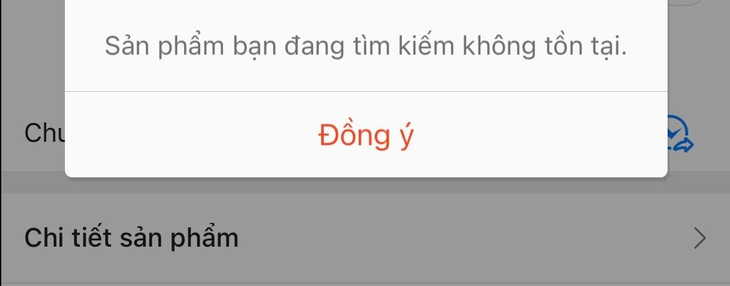
Notice that the product being searched for does not exist when clicking on a shop selling the product "Hong Thai nasal inhaler oil" - Screenshot
Mr. Teerapong said the company will work with the FDA to better understand the test results, while emphasizing that the company's previous internal tests during the product registration process have never found any abnormalities.
Hong Thai has now begun recalling product lot number 332 from the market. However, because this is an old production lot, only a part of it can be recalled.
“For distributors or customers who still have products from this batch, the company is willing to accept returns and refund 100%, and provide new replacement products with equivalent quantity without any additional costs,” Thai Herbal Hong Thai Ltd. said in the announcement.
The company plans to destroy the recalled products by November 4, 2025, in coordination with the FDA.
According to Tuoi Tre Online , the search results for "Hong Thai nasal inhaler" on a popular e-commerce platform in Vietnam are now fewer than before. Some shop owners have hidden or removed this item.
NGHI VU
Source: https://tuoitre.vn/hieu-dau-hit-mui-hong-thai-thong-bao-thu-hoi-200-000-san-pham-trong-lo-bi-nhiem-khuan-20251029100736615.htm


![[Photo] Hue: Inside the kitchen that donates thousands of meals a day to people in flooded areas](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/29/1761738508516_bepcomhue-jpg.webp)
![[Photo] Prime Minister Pham Minh Chinh chaired a meeting to evaluate the operation of the two-level local government model.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/29/1761751710674_dsc-7999-jpg.webp)


![[Photo] Prime Minister Pham Minh Chinh chaired a meeting to discuss solutions to overcome the consequences of floods in the central provinces.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/29/1761716305524_dsc-7735-jpg.webp)
![[Photo] Human love in the flood in Hue](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/10/29/1761740905727_4125427122470875256-2-jpg.webp)



































































![[Live] Concert Ha Long 2025: "Heritage Spirit - Brightening the Future"](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/10/29/1761743605124_g-anh-sang-am-thanh-hoanh-trang-cua-chuong-trinh-mang-den-trai-nghiem-dang-nho-cho-du-khach-22450328-17617424836781829598445-93-0-733-1024-crop-1761742492749383512980.jpeg)






























Comment (0)