On June 5, the Dong Nai Department of Health decided to revoke the validity of the certificate of registration of the declaration of nutritional formula products for 17 nutritional products announced by Latimum Gold Company Limited (Tan Binh Commune, Vinh Cuu District, Dong Nai). Accordingly, the 17 recalled products are all nutritional formula products for children from 1 to 17 years old .

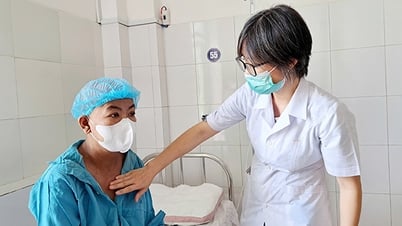
Latiplus Grow IQ milk is on the list of 17 products whose nutritional formula product registration certificates have been revoked (Photo: Latimum Gold Company).
Specifically, the formula nutritional products: Latiplus Grow IQ (for children 1-17 years old), Latiplus Pedia Plus (for children 1-12 years old), Latiplus Colostrum (for children 0-12 years old), Latiplus Colostrum (for children 1-3 years old), Latiplus Gain Kids (for children 1-12 years old), Latimum Gold Colostrum 2 (for children 1-3 years old), Latimum Gold Colostrum 1 (for children 0-12 months old), Latimum Gold Pedia Plus (for children 1-12 years old), Latimum Gold Naturegold (for children 1 year old and up), Latimum Gold Grow IQ (for children 1-17 years old), Latimum Gold Gain Kids (for children 1-12 years old), Latidairy Gold Gain Kids (for children 1-12 years old), Latidairy Gold Grow IQ (for children 1-17 years old), Latidairy Gold Naturegold (for children 1 year old and up), Latidairy Gold Colostrum 2 (for children 1-3 years old), Latidairy Gold Pedia Plus (for children 1-12 months old) and Latidairy Gold Colostrum (for children 0-12 months old).
Previously, on May 26, Latimum Gold Company Limited sent a document to Dong Nai Department of Health requesting to revoke the validity of the product declaration registration certificate. Recently, this company has submitted the declaration documents to the Department of Food Safety and Hygiene (now the Department of Food Safety and Hygiene, Department of Health).
In a document sent to the Department of Health, a representative of Latimum Gold Company Limited explained that the reason for returning the product declaration receipt was due to ineffective business operations. The company committed to stopping the sale of the above products.
In addition, the company also has a document requesting to cancel the self-declaration of 6 food supplement products (posted on the company's website from February 2022).
Specifically, Latiplus sure muscle - bone - joint supplement; Latiplus gain weight supplement; Latiplus calcium pro supplement; Latiplus diacerna supplement; Latiplus mama pro supplement; Latiplus colostrum supplement.
Source: https://dantri.com.vn/xa-hoi/mot-cong-ty-sua-o-dong-nai-bat-ngo-xin-dung-kinh-doanh-17-san-pham-20250605234520438.htm


























![[Photo] President Luong Cuong works with Hung Yen and Thai Binh Provincial Party Committees on implementing Resolution of the 11th Central Conference, 13th tenure](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/6/127b735d2761484d81dcee0d7725a25b)
![[Photo] General Secretary To Lam receives Korean Ambassador to Vietnam](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/6/6/a0765b7543784cbcbfe4755b67d43ab4)

























































![[OCOP REVIEW] Tu Duyen Syrup - The essence of herbs from the mountains and forests of Nhu Thanh](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/6/5/58ca32fce4ec44039e444fbfae7e75ec)











Comment (0)