At the afternoon meeting on November 21, the 8th session of the 15th National Assembly , with the majority of delegates in agreement, the National Assembly officially passed the Law amending and supplementing a number of articles of the Law on Pharmacy.

With 88.94% of delegates voting in favor, the Law amending and supplementing a number of articles of the Law on Pharmacy was officially passed at the 8th session of the 15th National Assembly.
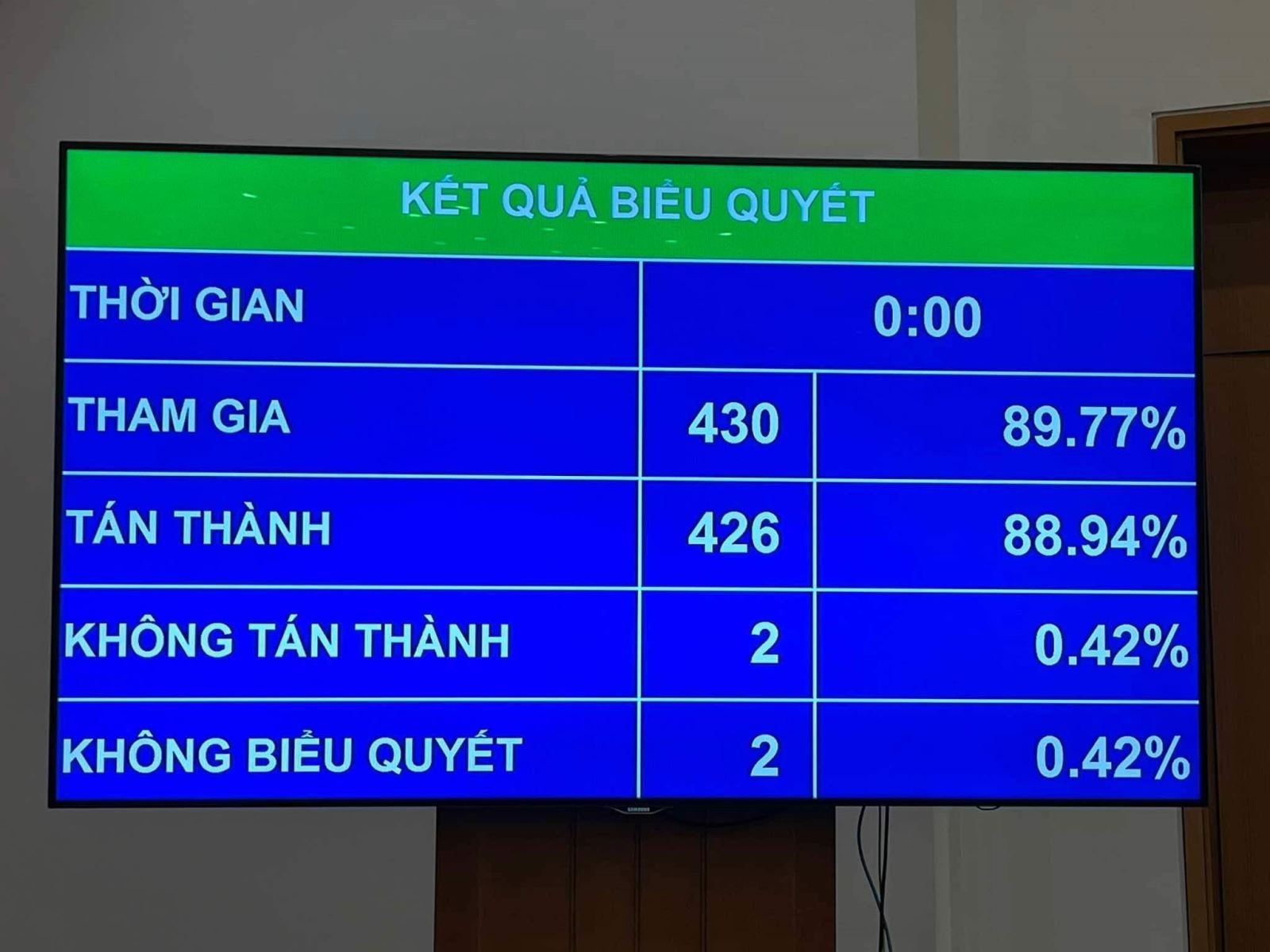
Before the National Assembly deputies voted to approve the entire draft Law amending and supplementing a number of articles of the Law on Pharmacy, the National Assembly voted to approve 2 contents of the Law including:
Regarding Clause 23, Article 1 on the rights and responsibilities of establishments organizing pharmacy chains and pharmacies in the pharmacy chain. The voting result was 89.14% of delegates in favor.
Vote on Clause 30, Article 1 on authority, records, procedures, time for granting and extending registration certificates for circulation of drugs and pharmaceutical ingredients. The voting result was 86.22% of delegates in favor.
Before voting, Ms. Nguyen Thuy Anh, Chairwoman of the National Assembly's Social Committee, read the report on receiving, explaining and revising the draft Law amending and supplementing a number of articles of the Law on Pharmacy.
Specifically, regarding the State's policy on pharmaceuticals and pharmaceutical industry development (Articles 7 and 8 (amended), taking into account the opinions of delegates, the National Assembly Standing Committee directed the review of Article 7 (amended) to ensure that it reflects the major content of principles; adding the provisions "There is a policy to control the number of drug circulation registration certificates for drugs with the same active ingredients and medicinal materials in accordance with the socio-economic conditions in each period" in Clause 14 and "Prioritize investment in developing information technology infrastructure, implementing digital transformation in pharmaceutical activities" in Clause 15. However, in order for the policy to come into practice, especially the provisions in Clauses 8, 13, 14, the National Assembly Standing Committee would like to ask for permission to still assign the Government to specify this Article in detail.
Regarding preferential policies and investment support in industrial development, based on the opinions of the majority of delegates, the National Assembly Standing Committee agreed with the Government to adopt Clause 1, Article 8 according to Option 1, which is to allow the application of special incentives and investment support to newly established projects in the pharmaceutical sector with a total investment capital of VND 3,000 billion or more, disbursing a minimum of VND 1,000 billion within 3 years from the date of issuance of the Investment Registration Certificate or approval of the investment policy. At the same time, to ensure consistency in preferential policies and special investment support in the pharmaceutical sector, the National Assembly Standing Committee requested the Government to direct attention to expressing tax incentives when amending tax laws and related laws, including the draft Law on Corporate Income Tax (amended).
Regarding pharmacy chain business (Article 17a (supplemented); Articles 31, 32, 33, 36, 37 and 38 (amended); Article 47a (supplemented); based on the opinions of delegates, the National Assembly Standing Committee reviewed and revised the provisions on pharmacy chains; including the content of assigning the Minister of Health to regulate the rotation of persons responsible for pharmaceutical expertise among pharmacies in the chain as shown in Point g, Clause 2, Article 47a (supplemented); regulating the responsibility of pharmacies in the chain to temporarily suspend operations in case the pharmacy chain organization facility temporarily suspends operations for 6 months or more (Point d, Clause 4, Article 47a (supplemented), and to terminate operations when the pharmacy chain organization facility ceases operations (Point d, Clause 4, Article 47a (supplemented).
Regarding the trading of drugs and pharmaceutical ingredients by e-commerce (Articles 6, 32 and 42 (amended)); based on the opinions of delegates, to ensure timely management of situations that may arise in practice, in addition to the rights and responsibilities prescribed in the draft Law, the Standing Committee of the National Assembly has added a clause stipulating that pharmaceutical business establishments by e-commerce must comply with the Government's regulations on wholesale of drugs, pharmaceutical ingredients, and retail of drugs by e-commerce at Point h, Clause 4, Article 42 (amended). At the same time, the provisions on the rights and responsibilities of pharmaceutical business establishments when trading by e-commerce are summarized in Clause 4, Article 42 (amended).
Regarding the issuance, extension, change and supplementation of Drug Circulation Registration Certificates (Article 56 (amended); implementing the principle of innovative thinking in legislative construction, the National Assembly Standing Committee has revised the amended content in Article 56 in the direction of not specifically regulating in the Law but assigning the Minister of Health to specify the dossiers and procedures for issuance, extension, change and supplementation of drug and pharmaceutical ingredient circulation registration certificates in Article 56.
In addition, accepting the opinions of delegates suggesting to study and supplement regulations to limit the issuance of new circulation registration certificates for drugs with overlap, to limit the exploitation of policies to "reserve" registration for circulation licenses due to low licensing fees, in the immediate future, Clause 7, Article 56 (amended) stipulates that the circulation registration certificate for drugs that have not been circulated on the market for 5 years will not be extended. At the same time, Clause 14, Article 7 (amended) stipulates that the State has a policy to control the number of drug circulation registration certificates for drugs with the same active ingredients and medicinal herbs and assigns the Government to provide detailed regulations.
Regarding drug price management (explanation of terms in clauses 44, 45, 46 and 47, Article 2 (amended); Articles 107, 109, 110, 112 and 113 (amended); repealing Article 114): In response to delegates' opinions, the National Assembly Standing Committee reviewed and agreed with the Government's proposal to regulate measures to announce expected wholesale prices applicable to prescription drugs. This is a specific measure in drug price management because prescription drugs account for a large proportion of the market, are widely used in medical facilities and patients must buy them according to the doctor's prescription. In addition, the Ministry of Health's regulations recommend expected wholesale drug prices to drug businesses to limit drug price increases through each level, intermediate level and increase prices when they reach consumers. At the same time, maintain current regulations on maximum retail margin for drugs sold at drug retail establishments within the premises of medical examination and treatment facilities.
Regarding the delegates' recommendations on price declaration measures, since this content is implemented according to the law on prices, the National Assembly Standing Committee proposed that the Government review the guiding documents and detailed regulations of the 2023 Law on Prices to supplement regulations on criteria for selecting the list of establishments required to declare prices, ensuring consistent and transparent implementation in localities; at the same time, closely monitor the implementation process, promptly detect difficulties and problems for handling; request the Ministry of Health to periodically update the List of Essential Medicines to ensure full guidance according to the provisions of the law on prices.
Regarding the implementation provisions (Article 3): In response to the opinions of delegates, the National Assembly Standing Committee has revised the implementation effect and transitional provisions as shown in Article 3 of the draft Law, accordingly, the Law takes effect from July 1, 2025 (Clause 1, Article 3) and only applies from January 1, 2025 to the provisions on extending the registration certificate for circulation of drugs and pharmaceutical ingredients, and the provisions on wholesale of drugs and pharmaceutical ingredients by drug manufacturing, importing and wholesaling establishments (Clause 2, Article 3) to immediately overcome difficulties in pharmaceutical business.
The opinions of the National Assembly deputies have been seriously studied, fully absorbed and the draft Law has been revised in specific contents such as: (i) the concepts of "non-prescription drugs", "high-tech drugs"; (ii) stipulating that the person responsible for pharmaceutical expertise of the establishment organizing the chain of pharmacies must have a pharmacist degree and have 02 years of professional practice at a suitable pharmaceutical establishment; (iii) ensuring transparency and consistency in regulations on the rights of manufacturing establishments, export and import establishments, wholesale establishments of drugs, pharmaceutical ingredients, and foreign-invested pharmaceutical establishments; (iv) allowing medical examination and treatment establishments to import drugs that do not have a circulation registration certificate in Vietnam to serve the special treatment needs of the establishment's patients at Point i, Clause 2, Article 60 and assigning the Government to specify in detail the transfer of drugs at Point d, Clause 7, Article 60; (v) Assign the Minister of Health to prescribe the form and method of drug information (instead of the Government as currently) and to revise the wording, style, and legislative techniques in most articles and clauses of the draft Law.
The revised draft Law consists of 3 articles, of which Article 1 amends 50 articles, abolishes 2 points, 2 clauses and 1 article of the current Pharmacy Law, and adds 3 new articles; Article 2 amends and supplements Appendix No. 01 issued with Price Law No. 16/2023/QH15; Article 3 on Implementation provisions.
The contents of reception, explanation and adjustment are specifically presented in Report No. 1062 with 06 major contents, 10 specific contents and other contents.
Compared with the current Law, the draft Law has 7 basic new groups of points as follows:
The State's policy on pharmaceuticals continues to be improved, institutionalizing the Party's viewpoints with the aim of developing the Vietnamese pharmaceutical industry into a spearhead industry. Accordingly, the draft Law supplements a number of more innovative provisions compared to the 2016 Pharmaceutical Law to attract investment and further promote research and development of drug and pharmaceutical ingredient production, such as preferential policies on administrative procedures when granting circulation registration certificates and import licenses; policies on applying incentive mechanisms and support from support funds for scientific and technological activities in research, development, clinical trials, technology transfer, and production of drugs and pharmaceutical ingredients; policies on price maintenance and price reduction for a number of drug groups with production technology transfer; policies on digital transformation in pharmaceutical activities; Determine the scale of projects in the pharmaceutical sector that are eligible for incentives and special investment support, and assign the Government to provide detailed regulations to ensure feasibility and bring the State's policies on pharmaceuticals and pharmaceutical industry development into life.
Creating a legal corridor for new business forms and methods, namely: (i) regulating the establishment of a pharmacy chain as a separate type of pharmaceutical business establishment, business conditions, rights and responsibilities of the establishment of a pharmacy chain, pharmacies in the pharmacy chain, notably the right to rotate drugs and the right to rotate people responsible for pharmaceutical expertise between pharmacies in the pharmacy chain; (ii) regulating the trading of drugs and pharmaceutical ingredients by e-commerce, specifically, supplementing regulations on electronic means, types of drugs and pharmaceutical ingredients allowed to be traded by e-commerce; supplementing the rights and responsibilities of pharmaceutical business establishments by this method.
Specify the rights and responsibilities of foreign-invested pharmaceutical businesses in the Law to ensure publicity and transparency in state management.
Expand the rights of manufacturing establishments, export and import establishments, and drug and pharmaceutical ingredient wholesale establishments to sell directly to a number of medical facilities, drug rehabilitation facilities, testing facilities, research and training facilities, and a number of other facilities; allow medical examination and treatment facilities to import drugs to serve the special treatment needs of patients at the medical examination and treatment facilities.
Promote administrative procedure reform in drug and pharmaceutical ingredient registration in the direction of classifying drugs and pharmaceutical ingredients based on different levels of drug properties as well as circulation to adjust records, procedures, time limits for granting, extending, changing, and supplementing drug and pharmaceutical ingredient registration certificates accordingly to increase the ability to access drugs early for people while still ensuring the effectiveness of state management, quality control, safety and effectiveness of drugs; supplement regulations to limit the issuance of duplicate registration numbers. At the same time, there are specific regulations on records, procedures for drug and pharmaceutical ingredient registration, drug testing and drug business in general to meet the requirements of national defense, security, natural disasters, catastrophes and epidemics.
Abolish the procedure for confirming drug information content. Strengthen decentralization, delegation of authority, promote the role of the Department of Health in recalling and promptly handling drugs that violate quality in the management area, ensuring safe and effective use of drugs.
Prescribe price management measures to comply with the Law on Prices and specific measures in drug price management are to announce and re-announce expected wholesale prices applicable to prescription drugs, ensuring that drug wholesale through intermediary levels does not exceed the announced expected wholesale prices.
VNA
Source: https://baohanam.com.vn/chinh-tri/nguoi-dai-bieu-nhan-dan/quoc-hoi-chinh-thuc-thong-qua-luat-duoc-sua-doi-140545.html




![[Photo] Prime Minister Pham Minh Chinh and Prime Minister of the Kingdom of Thailand Paetongtarn Shinawatra attend the Vietnam-Thailand Business Forum 2025](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/16/1cdfce54d25c48a68ae6fb9204f2171a)
![[Photo] President Luong Cuong receives Prime Minister of the Kingdom of Thailand Paetongtarn Shinawatra](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/16/52c73b27198a4e12bd6a903d1c218846)


















![[Photo] The Prime Ministers of Vietnam and Thailand witnessed the signing ceremony of cooperation and exchange of documents.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/16/935407e225f640f9ac97b85d3359c1a5)

































































Comment (0)