Specifically, the list of counterfeit drugs includes:
- Clorocid TW3 tablets (Cloramphenicol 250mg), Registration number: VD-25305-16, Manufacturer: TW 3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
- Tetracycline TW3 tablets (Tetracycline hydrochloride 250mg), Registration number: VD-28109-17, Manufacturer: TW 3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
- Pharcoter tablets (Codein base 10mg; Terpin hydrate 100mg), Registration number: VD-14429-11, Manufacturer: Central Pharmaceutical Joint Stock Company 1 (Pharbaco), packaged in plastic bottles of 400 tablets.
- Counterfeit Neo-Codion drug product (Neo-Codion drug is licensed for circulation by the Ministry of Health with the following information: Circulation license number: 300111082223 (Old registration number: VN-18966-15); Active ingredient: Codein base (as Codein camphosulfonate 25mg) 14.93mg; Sulfogaiacol 100mg; Grindelia soft extract 20mg; Dosage form: Sugar-coated tablets; Packaging: Box of 2 blisters x 10 tablets; Manufacturer: Sophartex Company (France), address: 21, rue du Pressoir, Vernouillet, 28500).
According to Mr. Ta Manh Hung, Deputy Director of the Department of Drug Administration, these are 4/21 counterfeit products that the drug counterfeiters have licensed for circulation by the Ministry of Health .
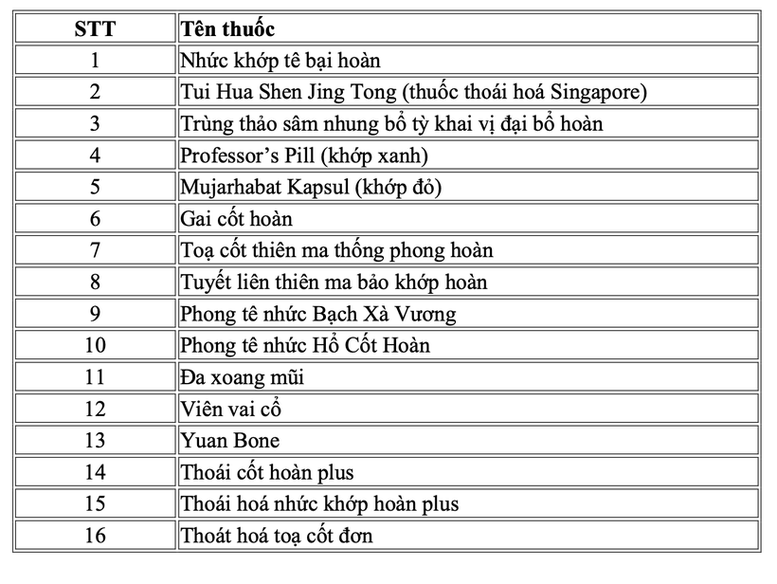
16 products not on the list of drugs that have been granted circulation registration by the Ministry of Health, including:
Require hospitals to review drug supply activities
The Drug Administration of Vietnam requests the Departments of Health to strictly implement Official Dispatch No. 41/CD-TTg dated April 17, 2025 on handling cases of production and trade of counterfeit medicines and health protection foods; Directive 17/CT-TTg dated June 19, 2018 of the Prime Minister on strengthening the fight against smuggling, trade fraud, production and trade of counterfeit and poor-quality goods in the group of pharmaceuticals, cosmetics, functional foods, medicinal herbs and traditional medicine ingredients...
At the same time, direct medical examination and treatment facilities to review the drug purchasing and supply process and the drug supply situation in the past; ensure that the drugs supplied are licensed for circulation and supplied from legal pharmaceutical businesses, with full invoices and documents.
In case of detecting drugs with signs of suspected counterfeiting or drugs that have not been licensed for circulation, immediately seal the drugs, do not continue to use the drugs and report to the health management agency and competent authorities for inspection, verification and handling according to the provisions of law.
The Departments of Health also need to coordinate with media agencies and press to inform drug trading and usage establishments and people not to trade or use the above counterfeit drugs; only buy and sell drugs at legal pharmaceutical businesses; do not buy and sell drugs of unknown origin; report any suspicious signs of production and trading of counterfeit drugs or drugs of unknown origin to health agencies and competent authorities.
At the same time, it is necessary to strengthen inspection and examination of pharmaceutical businesses, wholesale and retail establishments, and drug users in the area, focusing on checking the origin of drugs sold and used by businesses; direct the Testing Center to increase sampling and quality testing of drugs circulating in the area with drugs at risk of being counterfeited or of poor quality...
Hien Minh
Source: https://baochinhphu.vn/danh-sach-thuoc-gia-bo-y-te-yeu-cau-khong-duoc-kinh-doanh-buon-ban-102250419230347139.htm






![[Photo] Top players gather at the 2025 Nhan Dan Newspaper National Table Tennis Championship](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/23/9ad5f6f4faf146b08335e5c446edb107)
![[Photo] Anh Hoang - Dinh Duc successfully defended the men's doubles championship of the National Table Tennis Championship of Nhan Dan Newspaper](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/23/d6ab3bcac02c49928b38c729d795cac6)




![[Video] Launch of the publication "The Teacher and the Books"](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/23/f8b7526776a44a57b0d4d9213c0c660d)

















































































Comment (0)