On November 13, the Drug Administration of Vietnam ( Ministry of Health ) sent an official dispatch to the health departments of provinces and cities and Dai Cat A International Company Limited (HCMC) regarding the suspension of circulation, recall and destruction of violating cosmetics.
In the official dispatch, the Drug Administration Department announced the suspension of circulation and nationwide recall of 22 cosmetic products of Dai Cat A International Company Limited - the organization responsible for bringing the products to the market (business address declared on the declaration file: in Phu Nhuan District, Ho Chi Minh City).
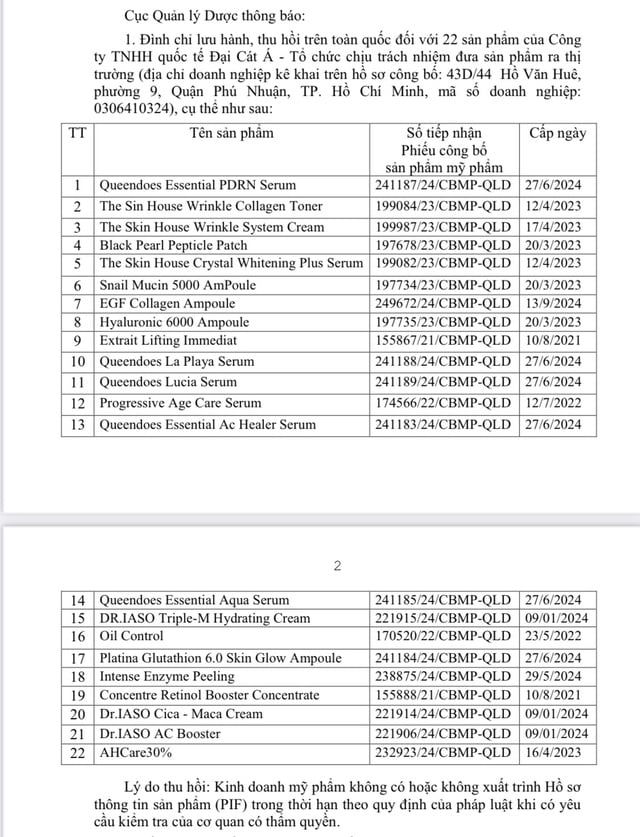
The Drug Administration of Vietnam announced the suspension of circulation and nationwide recall of 22 cosmetic products.
PHOTO: DAV.GOV.VN
Reason for recall: Cosmetics business does not have or does not present product information file (PIF) within the time limit prescribed by law when requested for inspection by competent authority.
The recalled cosmetics are products labeled: Queendoes essential PDRN serum, The Sin house wrinkle collagen toner, Hyaluronic 6000 ampoule, The Skin house crystal whitening plus serum; Snail mucin 5000 AmPoule, Queendoes lucia serum, DR.IASO triple-m hydrating cream...
The Drug Administration Department requests Dai Cat A International Company Limited to send recall notices to distributors and users of the 22 products mentioned above; receive returned products from business establishments and recall all 22 products that do not meet regulations for product destruction; send a recall report of the 22 cosmetic products mentioned above to the Drug Administration Department before November 25, 2025.
Propose that the Ho Chi Minh City Department of Health supervise Dai Cat A International Company Limited in implementing the recall and report the supervision results to the Department of Drug Administration before December 5, 2025.
The Department of Health of provinces and cities shall notify cosmetic businesses and users in the area to immediately stop selling and using the above 22 products and return them to the suppliers; recall the 22 violating products and inspect and supervise the implementing units; and handle violators according to current regulations.
Source: https://thanhnien.vn/dinh-chi-luu-hanh-thu-hoi-tren-toan-quoc-22-my-pham-185251113182512056.htm







![[Photo] Deep sea sand deposits, ancient wooden ship An Bang faces the risk of being buried again](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/13/1763033175715_ndo_br_thuyen-1-jpg.webp)
























































































![Dong Nai OCOP transition: [Article 3] Linking tourism with OCOP product consumption](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/11/10/1762739199309_1324-2740-7_n-162543_981.jpeg)







Comment (0)