Specifically, the Drug Administration Department has requested the suspension of circulation and recall of 7 cosmetic products announced and marketed by Dong Nam Global Pharmaceutical Company Limited (address: No. 12, Lane 40, Nguyen Chinh, Tan Mai Ward, Hoang Mai District, Hanoi ).
The reason for the recall was determined to be that the product formula did not match the approved declaration file.
 |
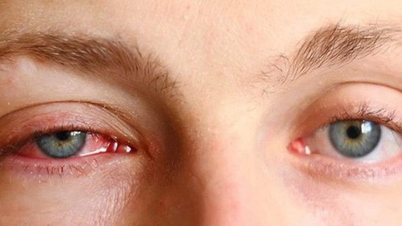
| Illustration photo. |
The 7 recalled products include: ME LINE 01 CAUCASIAN SKIN (registration number 243285/24/CBMP-QLD, issued on July 11, 2024), INNOAESTHETICS INNO-TDS XEROSKIN-ID (234983/24/CBMP-QLD, dated May 5, 2024),
INNOAESTHETICS INNO-DERMA DARK SPOT ERASER 24H CREAM (234976/24/CBMP-QLD, May 5, 2024), INNOAESTHETICS INNO-EXFO REDNESS PEEL (240521/24/CBMP-QLD, June 6, 2024),
INNOAESTHETICS INNO-EXFO TCAGE (247909/24/CBMP-QLD, August 20, 2024), INNOAESTHETICS INNO-EXFO SKIN RECOVERY (242690/24/CBMP-QLD, July 7, 2024) and ME LINE 02 CAUCASIAN SKIN NIGHT (236277/24/CBMP-QLD, May 16, 2024).
The Drug Administration of Vietnam has requested the Department of Health of provinces and cities to notify cosmetic businesses and users in the area about the suspension of circulation of the above products.
At the same time, the relevant units are required to immediately stop trading and using and recall all products. The Department also requires Dong Nam Global Pharmaceutical Company Limited to send a recall notice to distribution facilities, recall all products sold and handle them in accordance with current regulations.
Notably, the Drug Administration has decided to temporarily suspend the review and acceptance of new cosmetic product declaration dossiers of Dong Nam Global Pharmaceutical Company Limited for a period of 6 months. The dossiers submitted before the date of the decision are also no longer valid for processing.
Also on the same day, the Drug Administration continued to issue a decision to suspend circulation and recall two cosmetic products due to violations of labeling regulations.
The two recalled products are Aquafresh Soft Mint (registration number 264128/25/CBMP-QLD, issued on January 22, 2025) and Aquafresh Clear Mint (264127/25/CBMP-QLD, issued on January 22, 2025), announced and marketed by Phat Anh Minh Company Limited (located in Tu Hiep commune, Thanh Tri district, Hanoi). The reason for the recall is that the product labels do not meet current regulations on cosmetic labeling.
The Drug Administration of Vietnam requires the Departments of Health to monitor the recall process, strictly handle violations according to regulations, and report the results to the Department for synthesis and monitoring.
This is a move to strengthen the control of cosmetic quality in the market, ensuring the rights and safety of consumers. The Drug Administration recommends that organizations and individuals absolutely do not use suspended products, and proactively coordinate with competent authorities to recall and destroy substandard products.
Source: https://baodautu.vn/dinh-chi-luu-hanh-va-thu-hoi-nhieu-my-pham-vi-pham-quy-dinh-d325654.html


![[Photo] Dan Mountain Ginseng, a precious gift from nature to Kinh Bac land](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F30%2F1764493588163_ndo_br_anh-longform-jpg.webp&w=3840&q=75)







































































































Comment (0)