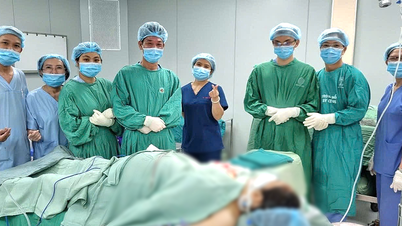
Cell therapy poses major challenges in management, science , and research ethics - Photo: Provided by the hospital
On the morning of October 6, at the Pasteur Institute in Ho Chi Minh City, the Department of Science, Technology and Training, Ministry of Health organized a workshop on developing national standards (TCVN) for cells and stem cell products used in research.
The event brings together leading managers, scientists, and experts from research institutes, universities, and businesses in the fields of biotechnology and medicine; to discuss and contribute ideas to build a legal, technical, and quality corridor for research and application of cell therapy in Vietnam.
People spend large sums of money to use "hand-carried cells" of unknown quality.
At the workshop, Mr. Nguyen Ngo Quang - Director of the Department of Science, Technology and Training, Ministry of Health - said that cell technology and cell products in the medical field are identified as strategic technologies, prioritized for development in the coming period.
The Ministry of Health has reported to the Secretariat and this content has been included in the Prime Minister's decision, to encourage the participation of scientists, production facilities and investors.
In Vietnam, the field of cells and cell products is still very new, and no official major has been formed. Many scientists, facilities and units have seriously invested, complied with regulations, and focused on ensuring the quality of cells and cell products in research and application.
However, in recent times, the development of the cell field has lacked control. Many medical facilities, clinics, and beauty salons (both official and unofficial) have taken advantage of this trend to apply cells to customers without control, causing many health consequences.
A burning issue is that products called "stem cells" are brought into Vietnam by hand, without quality control documents but are still widely advertised to treat many diseases. People have to spend a lot of money on products of unknown origin and quality.
This situation creates unfair competition with medical facilities and scientists who have invested properly in technology, equipment, quality control processes and published facility standards.
In 2025, the Ministry of Health will issue national standards on cells and cell products.
Mr. Quang added that, in addition to the regulations in the Law on Medical Examination and Treatment and the circulars and decrees under the law, the issue of controlling the quality of cells and cell products before using them on humans is extremely important.
To control the quality of cells and cell products, they must go through 8 very detailed sets of standards, from receiving samples, signing contracts to performing specialized tests and returning results.
In 2025, the Ministry of Health will issue national standards (TCVN) on cells and cell products, initially applied in research and clinical applications. In the next phase, these standards will be deployed in treatment practice.
"Cell technology and cell products in the medical field are the priority strategy of the Ministry of Health from now until 2035," Mr. Quang concluded.
A representative of the Pasteur Institute in Ho Chi Minh City said that to ensure objectivity before a cell product is put into clinical trials, an independent testing unit is needed to evaluate the quality according to strict criteria.
The Institute recommends that the Ministry of Health provide guidance on testing cells used in research, selecting the most important criteria for identification, efficacy and safety for uniform application.

Deputy Minister of Health Nguyen Tri Thuc - Photo: Provided by Pasteur Institute of Ho Chi Minh City
In his speech, Deputy Minister of Health Nguyen Tri Thuc said that this is a very special event, aiming to listen to reports and comments from scientists, experts, as well as from production facilities and clinical practices.
The Deputy Minister affirmed that the first set of standards plays an important role, serving as the foundation for the entire following process, aiming to concretize the Politburo's resolutions on science and technology development and people's health care, while meeting the requirements of the law on standards and quality, which will take effect from January 2026.
"The core goal of building TCVN is to establish a national standards system to serve scientific research, moving towards application in treatment with the ultimate goal of protecting people's health.
At the same time, it helps standardize research processes and treatment regimens, avoiding unfortunate risks that may occur when widely deployed without a unified direction from the Ministry of Health," Deputy Minister Nguyen Tri Thuc emphasized.
Source: https://tuoitre.vn/nhuc-nhoi-thuc-trang-dung-te-bao-troi-noi-bo-y-te-se-ban-hanh-bo-tieu-chuan-trong-nam-2025-20251006115309451.htm






![[Photo] Dan Mountain Ginseng, a precious gift from nature to Kinh Bac land](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F30%2F1764493588163_ndo_br_anh-longform-jpg.webp&w=3840&q=75)































































































Comment (0)