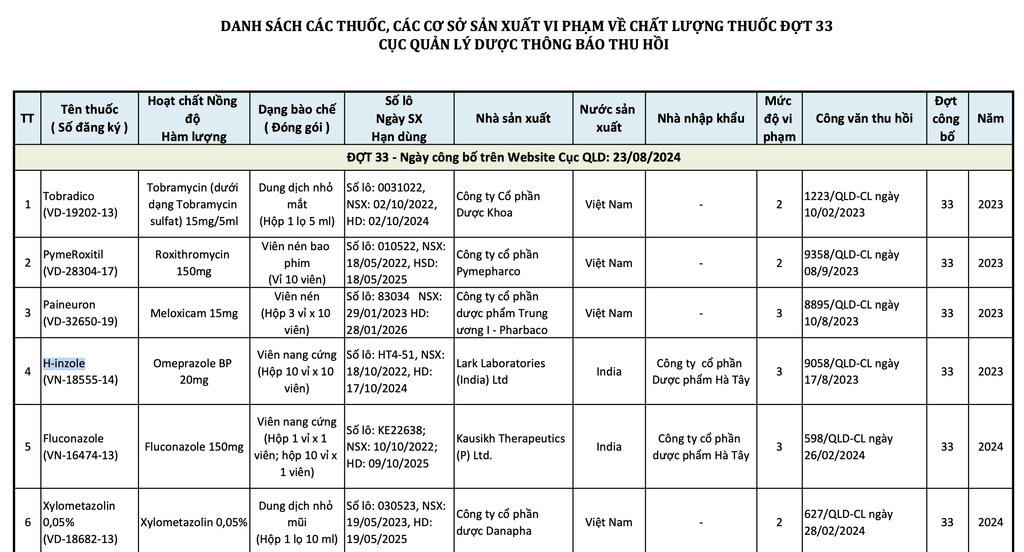
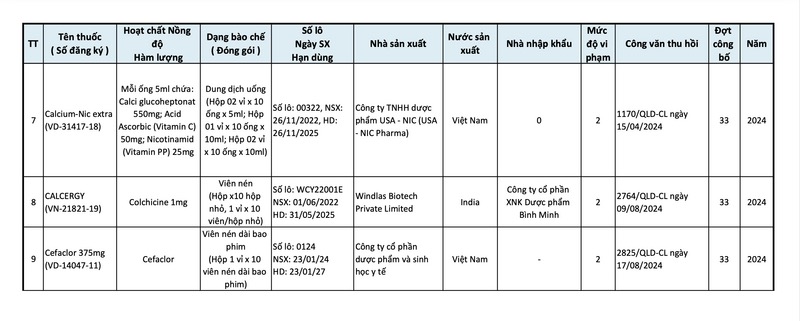
Accordingly, this list includes 9 types of drugs and manufacturing facilities violating drug quality in the 33rd batch announced by the Drug Administration for recall, updated from February 10 to August 17:
Tobradico eye drops (VD-19202-13): Content: Tobramycin (as Tobramycin sulfate) 15mg/5ml; Batch number: 0031022; Expiry date: October 2, 2024; Manufacturer: Vietnam Pharmaceutical Joint Stock Company.
PymeRoxitil (VD-28304-17): Content: Roxithromycin 150mg; Expiry date: May 18, 2025; Manufacturer: Pymepharco Vietnam Joint Stock Company.
Paineuron tablets (VD-32650-19): Content: Meloxicam 15mg; Batch number: 83034; Expiry date: 28.1.2026; Manufacturer: Central Pharmaceutical Joint Stock Company I - Pharbaco Vietnam.
In addition, 6 types of drugs including: H-Inzole hard capsules, Fluconazole hard capsules manufactured by Ha Tay Pharmaceutical Joint Stock Company; Nasal drops manufactured by Danapha Vietnam Pharmaceutical Joint Stock Company, etc. were also recalled in this batch.
Below is the complete list of drugs and manufacturing facilities that violated drug quality regulations from the 33rd recall announcement by the Drug Administration of Vietnam - Ministry of Health :
Source: https://laodong.vn/y-te/9-loai-thuoc-vi-pham-ve-chat-luong-bi-bo-y-te-thu-hoi-1386558.ldo




![[Photo] Prime Minister Pham Minh Chinh meets with Hungarian President Sulyok Tamas](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/29/dbcaa73e92ea4448a03fe1d0de6d68e8)


![[Photo] Vietnamese and Hungarian leaders attend the opening of the exhibition by photographer Bozoky Dezso](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/29/94d8ceca5db14af3bf31285551ae4bb3)



















































































Comment (0)