Specifically, the Drug Administration received a report from the Hanoi Center for Drug, Cosmetic and Food Testing on the test results of a drug sample labeled: DIAMICRON® MR 60mg (Gliklazid), batch number: 23F603, expiry date: 4-2026. This drug sample did not meet the quality standards for the quantitative index of the active ingredient Gliclazid according to the Vietnamese Pharmacopoeia, only reaching 70.83% compared to the content stated on the label.

In addition, the authorities also took 6 other drug samples from Duc Anh Pharmacy (belonging to Duc Anh Pharmaceutical and Medical Equipment Company Limited, No. 08 Huynh Thuc Khang Street, Lang Thuong Ward, Dong Da District, Hanoi) to test the quality. The results showed that all 6 drug samples had no information about the circulation registration number or import license, manufacturing facility and importing facility. The specific products include:
- Oseltamivir, batch number: M1164B01, Manufacturing date: 3-2021, Expiry date: 3-2023
- Crestor 20mg (Rosuvastatin), lot number: A23237030, Expiry date: 4-2026
- Janumet 50/1000mg (Sitagliptin/Metformin), batch number: 24497505A, Expiry date: 7-2026
- Plavix (Clopidogrel), lot number: ELB04027, Expiry date: 5-2027
- NEXIUM® 40mg (Esomeprazole), lot number: 23H420, Expiry date: 9-2027
- Crestor 10mg (Rosuvastatin), lot number: A24236004, Expiry date: 7-2027
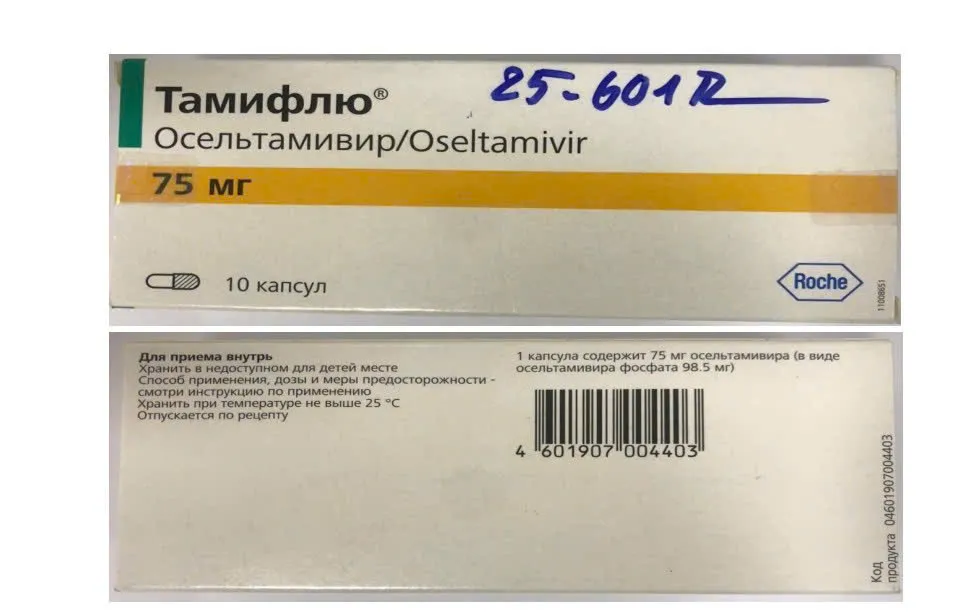
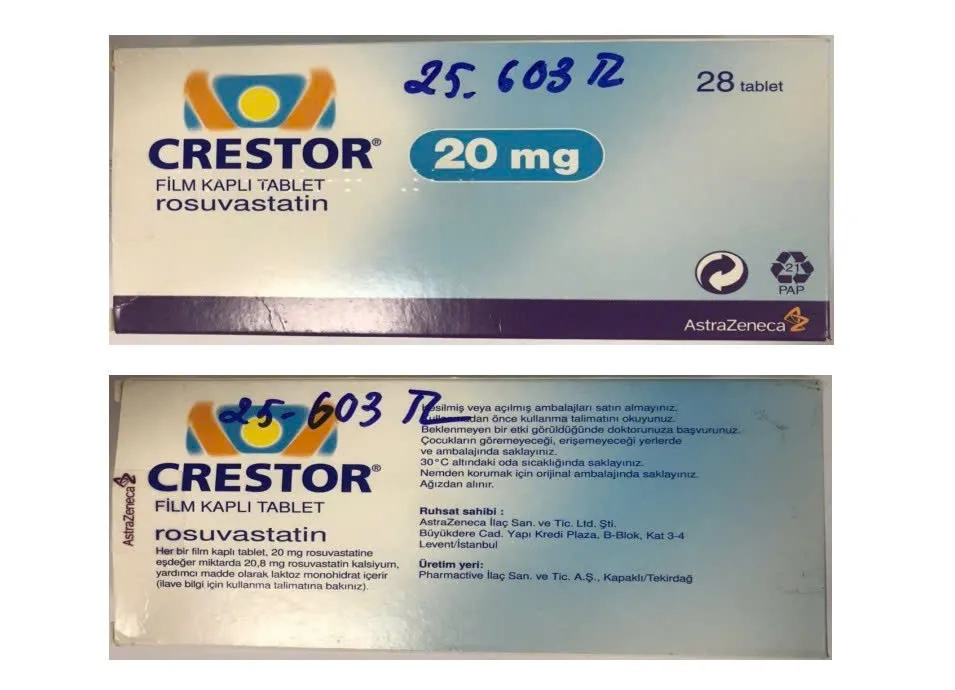

Faced with the above situation, the Drug Administration of Vietnam requested the Hanoi Department of Health to report to the Hanoi Steering Committee 389, coordinate with the police, market management agencies and related units to urgently inspect and examine the operations of Duc Anh Pharmacy, clarify the origin of 7 batches of drugs in violation, strictly handle them according to regulations and report the results to the Department before June 2.
The Department also requested the Department of Health of provinces and cities to direct drug businesses and users not to buy, sell or use the above products; at the same time, increase communication so that people are vigilant, only buy drugs at legal establishments, and promptly report suspicious signs of fake drugs or drugs of unknown origin to the authorities.
Source: https://www.sggp.org.vn/phat-hien-nhieu-loai-thuoc-gia-khong-ro-nguon-goc-tai-ha-noi-post797410.html



![[Photo] General Secretary To Lam receives Chief of the Central Office of the Lao People's Revolutionary Party](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/30/140435f4b39d4599a3d17975dfb444c5)
![[Photo] National Conference "100 years of Vietnamese Revolutionary Press accompanying the glorious cause of the Party and the nation"](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/30/1cf6cd5c8a934ebfa347028dcb08358c)







![[Video] Ho Chi Minh City: Number of COVID-19 cases increases rapidly, 2 deaths recorded](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/31/5fe289cf72774d918f23fe88bb1686ce)












![[Photo] Journalists moved to tears at the Memorial Service for the soldiers who died in Gac Ma](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/30/9454613a55c54c16bf8c0efa51883456)


































































Comment (0)