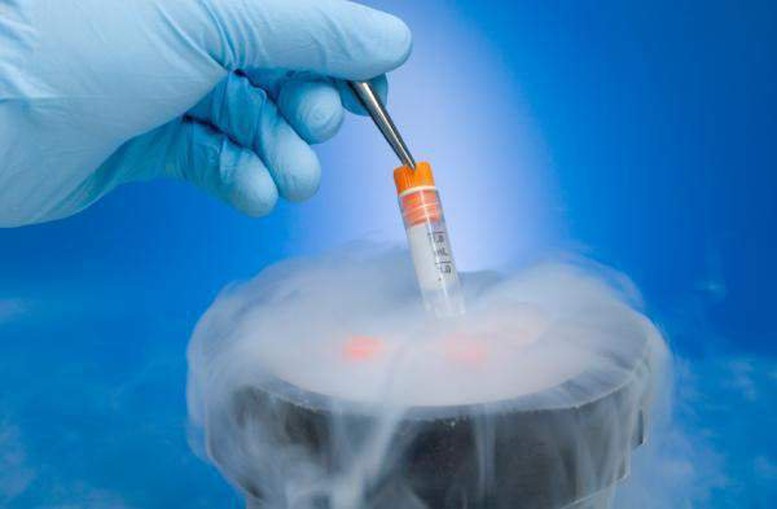
The facility receiving sperm, eggs, and embryos (samples) is responsible for considering the wishes of the sample sender/recipient and deciding to accept samples transferred from other storage facilities.
This Circular stipulates health standards for recipients of sperm, eggs, and embryos; the delivery and receipt of sperm, eggs, and embryos between facilities storing sperm, eggs, and embryos; the minimum list of techniques in medical examination and treatment that facilities performing in vitro fertilization must perform; statistical reporting, data management, and information sharing on reproductive support.
Health standards for sperm, egg, and embryo recipients
According to the Circular, the recipient of sperm, eggs, and embryos must not have any diseases or abnormalities of the reproductive organs to the extent that they cannot become pregnant; diseases that, during pregnancy, can endanger the woman's life; mental illness or other diseases that make it impossible to perceive or control one's behavior.
People with acute illnesses should delay embryo transfer until treatment is stable.
The head of the facility performing in vitro fertilization or a legally authorized person is responsible for organizing and conducting examinations, consultations, and concluding on health conditions to receive sperm, eggs, and embryos according to the above regulations.
Delivery and receipt of sperm, eggs, and embryos between facilities
In addition, the Circular regulates the delivery and receipt of sperm, eggs, and embryos between sperm, egg, and embryo storage facilities.
In case the person sending the sperm, egg, embryo (referred to as sample) or the person receiving the donated sample wishes to transfer the sample from the current storage facility to another storage facility:
The facility holding the sample advises the donor/recipient of the sample on the regulations for transporting the sample, the risks that may be encountered during the transport process, and gives written consent to transfer the sample.
The sample receiving facility is responsible for considering the wishes of the sample sender/recipient and deciding to accept samples transferred from other storage facilities. Delivery and receipt can only be carried out with the written consent of the sample receiving facility.
The transportation of samples must be carried out by medical staff of the receiving facility or the delivery facility with the participation of the sender/recipient of the donated sample or a legally authorized person. If the sender/recipient of the donated sample or a legally authorized person is not present, the sender/recipient of the donated sample must authorize the receiving facility or the delivery facility to carry out the transportation of the sample.
The cost of transporting the sample is paid by the sender/receiver of the donated sample. During transport, the sample must be kept in a specialized, sealed freezer by the sample delivery facility.
The sample receiving facility is responsible for checking the seal and accompanying documents before signing the sample handover minutes and returning an original copy to the delivering party.
This Circular takes effect from October 1, 2025.
Minh Hien
Source: https://baochinhphu.vn/quy-dinh-tieu-chuan-suc-khoe-cua-nguoi-nhan-tinh-trung-noan-phoi-10225081416132671.htm







![[Photo] Closing of the 14th Conference of the 13th Party Central Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/06/1762404919012_a1-bnd-5975-5183-jpg.webp)




























































































Comment (0)