
Perspective of VNVC Vaccine and Biological Factory expected to be operational in 2027
It is expected that this factory will come into operation by the end of 2027, gradually receiving technology transfer from Sanofi (France) to "localize" the production of important vaccines and new technology vaccines of Sanofi, serving the vaccination needs in Vietnam and participating in the global supply chain.
This event took place one day after VNVC Vaccine Company and Sanofi Group (France) exchanged cooperation documents on vaccine production technology transfer, witnessed byPresident Luong Cuong and French President Emmanuel Macron.
The groundbreaking ceremony of the VNVC Vaccine and Biological Products Factory is an important milestone in realizing the comprehensive strategic partnership between France and Vietnam in the fields of medicine and biotechnology, and is expected to make practical contributions to the common achievements of the two countries in the fields of medicine and health care.
At the same time, it demonstrates the strong determination of Vietnamese enterprises to proactively expand international cooperation, access and gradually master modern biomedical technologies, and proactively produce high-tech vaccines. Thereby, contributing to improving preventive medicine capacity, ensuring national health security in the face of the challenges of dangerous infectious diseases and new pandemics that have been predicted in the future.
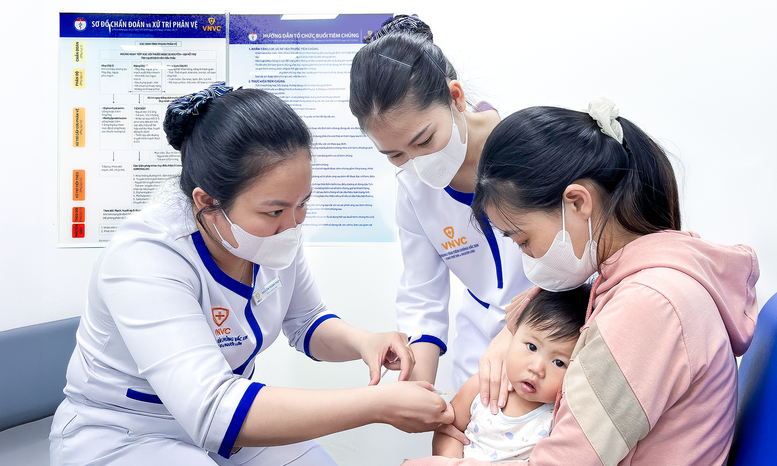
VNVC vaccine and biological factory is expected to have a capacity of up to 100 million doses/year.
Initial investment scale 2,000 billion VND
VNVC Vaccine and Biological Factory has an initial investment of about 2,000 billion VND, built on an area of over 26,000m² in Phu An Thanh Industrial Park, Ben Luc District, Long An Province. The factory is designed and operated according to the highest current standards of good manufacturing practices (GMP), including EU GMP (Europe), FDA GMP (USA) and WHO GMP ( World Health Organization).
At the same time, the Factory strictly meets the standards of good laboratory practice (GLP) and international standards (AAALAC) on safety, welfare and humane treatment for experimental animal research areas, in accordance with international scientific ethics principles and many other high standards on environmental protection, emission reduction, compliance with "green factory" and "smart factory" construction technology.
It is known that the factory will gradually produce important, high-quality vaccines of Sanofi, thereby fully meeting the large quantity of high-quality, low-cost "Make in Vietnam" vaccines. for the domestic market and towards export to countries in the region.
Attending the event, Mr. Burak Pekmezci, General Director of Sanofi Vietnam, expressed his joy and pride when witnessing the groundbreaking ceremony of the VNVC Vaccine and Biologicals Factory. Sanofi expects that the VNVC Global Standard Factory will soon provide advanced vaccines, medicines and effective disease prevention solutions to serve the people. This will be a breakthrough project, contributing to the mission of protecting the health of Vietnamese people better and better.
Capacity of 100 million vaccine doses/year
According to Mr. Ngo Chi Dung, Chairman of the Board of Directors and General Director of VNVC Vaccine Company, this is an inevitable need to master modern vaccine and biomedical pharmaceutical production technologies. At the same time, having a factory with a large capacity of hundreds of millions of vaccine doses per year will help reduce costs and be more proactive in supplying to domestic people, even for export.
From there, people will have equal access to new generation, effective and safe vaccines, and be fully vaccinated on schedule with good vaccines like people in advanced countries around the world.

Official groundbreaking ceremony of VNVC Vaccine and Biological Factory
In addition, the VNVC Vaccine and Biological Factory also invests in modern machinery and equipment infrastructure and has special policies to attract high-quality human resources in the world in the fields of scientific research, vaccine production and high-tech biomedical pharmaceuticals, thereby being ready to receive and master cutting-edge biomedical technologies, such as mRNA technology applied in the production of many new vaccines, especially expected to be vaccines for cancer treatment, Mr. Ngo Chi Dung emphasized.
VNVC Vaccine and Biological Factory was designed by Rieckermann Group (Germany) with over 130 years of experience in designing pharmaceutical, vaccine and biological factories. All equipment is imported genuine, new generation from Germany, USA... The factory has a capacity of up to 100 million doses of vaccine per year, initially with three modern filling and packaging lines, including advanced isolator technology and the first injection pen filling line in Southeast Asia, ensuring high quality and safety according to global standards.
The factory also invested in building a Research and Development Center (R&D) with a scale of 1,500 m², specializing in researching and applying advanced vaccine and biological product production technologies, especially mRNA technology vaccines.
The Center also plans to cooperate with scientists, universities, and global pharmaceutical companies to conduct preclinical and clinical trials right in Vietnam, helping Vietnam have early access to new innovative drugs and vaccines in the world, while meeting standards to shorten the time for product licensing registration to soon serve the people.
Hien Minh
Source: https://baochinhphu.vn/chinh-thuc-khoi-cong-nha-may-vaccine-va-sinh-pham-vnvc-102250527161259595.htm



![[Photo] 12th grade students say goodbye at the closing ceremony, preparing to embark on a new journey](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/28/42ac3d300d214e7b8db4a03feeed3f6a)
![[Photo] Prime Minister Pham Minh Chinh receives a bipartisan delegation of US House of Representatives](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/28/468e61546b664d3f98dc75f6a3c2c880)


![[Photo] Vietnamese and Hungarian leaders attend the opening of the exhibition by photographer Bozoky Dezso](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/28/b478be84f13042aebc74e077c4756e4b)























































































Comment (0)