Two products were recalled and destroyed nationwide - Photo: Department of Drug Administration
Dai Cat A International Co., Ltd., registered at 43D/44 Ho Van Hue, Ward 9, Phu Nhuan District, Ho Chi Minh City, is the entity that announced the above products. The list of recalled products includes many familiar names in the cosmetics market such as Queendoes, Dr.IASO or The Skin House.
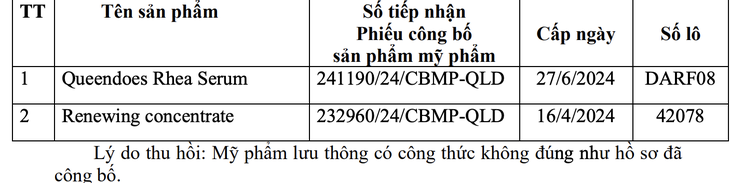
According to the Drug Administration, two products, Queendoes Rhea Serum and Renewing concentrate, were recalled due to their circulating formula not being consistent with the published records - a violation that could affect consumer safety.
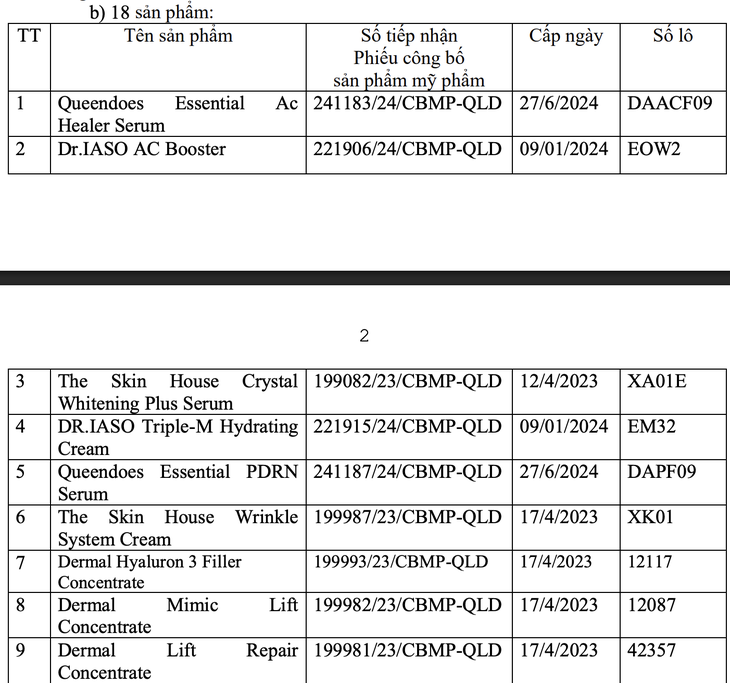
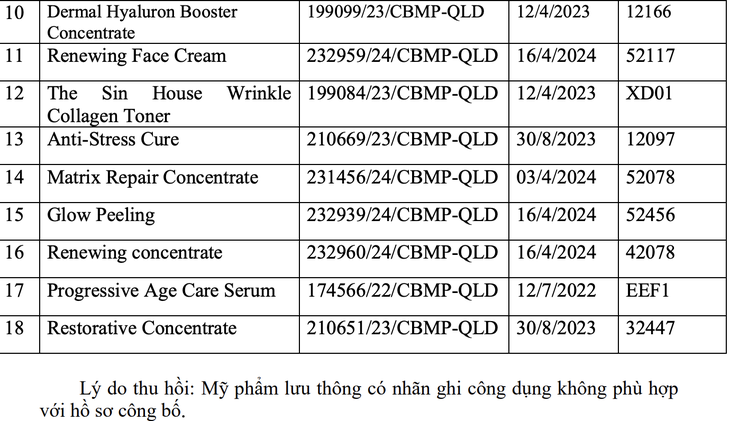
The remaining 18 products include Queendoes Essential Ac Healer Serum, Dr.IASO AC Booster, The Skin House Crystal Whitening Plus Serum, DR.IASO Triple-M Hydrating Cream, Queendoes Essential PDRN Serum, The Skin House Wrinkle System Cream, Dermal Hyaluron Filler3 Concentrate, Dermal Mimic Lift Concentrate;
Dermal Lift Repair Concentrate, Dermal Hyaluron Booster Concentrate, Renewing Face Cream, The Skin House Wrinkle Collagen Toner, Anti-Stress Cure, Matrix Repair Concentrate, AHCare 30%, Renewing concentrate;
Progressive Age Care Serum, Restorative Concentrate. Specifically, each batch was found to have inconsistent labeling with the published documents. This is a violation of regulations on advertising and publishing cosmetic products.
18 products recalled due to incorrect labeling - Photo: Department of Drug Administration
The Drug Administration Department requested the provincial and municipal health departments to notify businesses and cosmetic users to immediately stop selling, using and returning the 20 products mentioned above. At the same time, it must recall, monitor and handle units that do not comply with regulations.
As for Dai Cat A Company, the Department requires the company to send recall notices to distributors, receive returned products and recall all batches of products that do not meet regulations. With two products that violate the formula, the company must organize destruction.
For the remaining 18 products, if the violating element cannot be removed (for example, the incorrect product label cannot be separated from the packaging), they must also be destroyed according to the provisions of Decree 126/2021.
Dai Cat A must send a recall report of violating products to the Drug Administration before June 30, 2025. Along with that, the Ho Chi Minh City Department of Health is assigned to monitor the entire recall process, handle violations of the enterprise and report the results before July 10, 2025.
Consumers are advised to proactively check the product name, production batch and origin before use. Using products that do not guarantee the formula and declared uses can be harmful to skin health, even leading to serious reactions.
Source: https://tuoitre.vn/thu-hoi-20-loai-my-pham-cua-cong-ty-dai-cat-a-chuyen-nhap-my-pham-o-tp-hcm-20250622105607717.htm




![[Photo] Da Nang: Hundreds of people join hands to clean up a vital tourist route after storm No. 13](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/07/1762491638903_image-3-1353-jpg.webp)



































































































Comment (0)