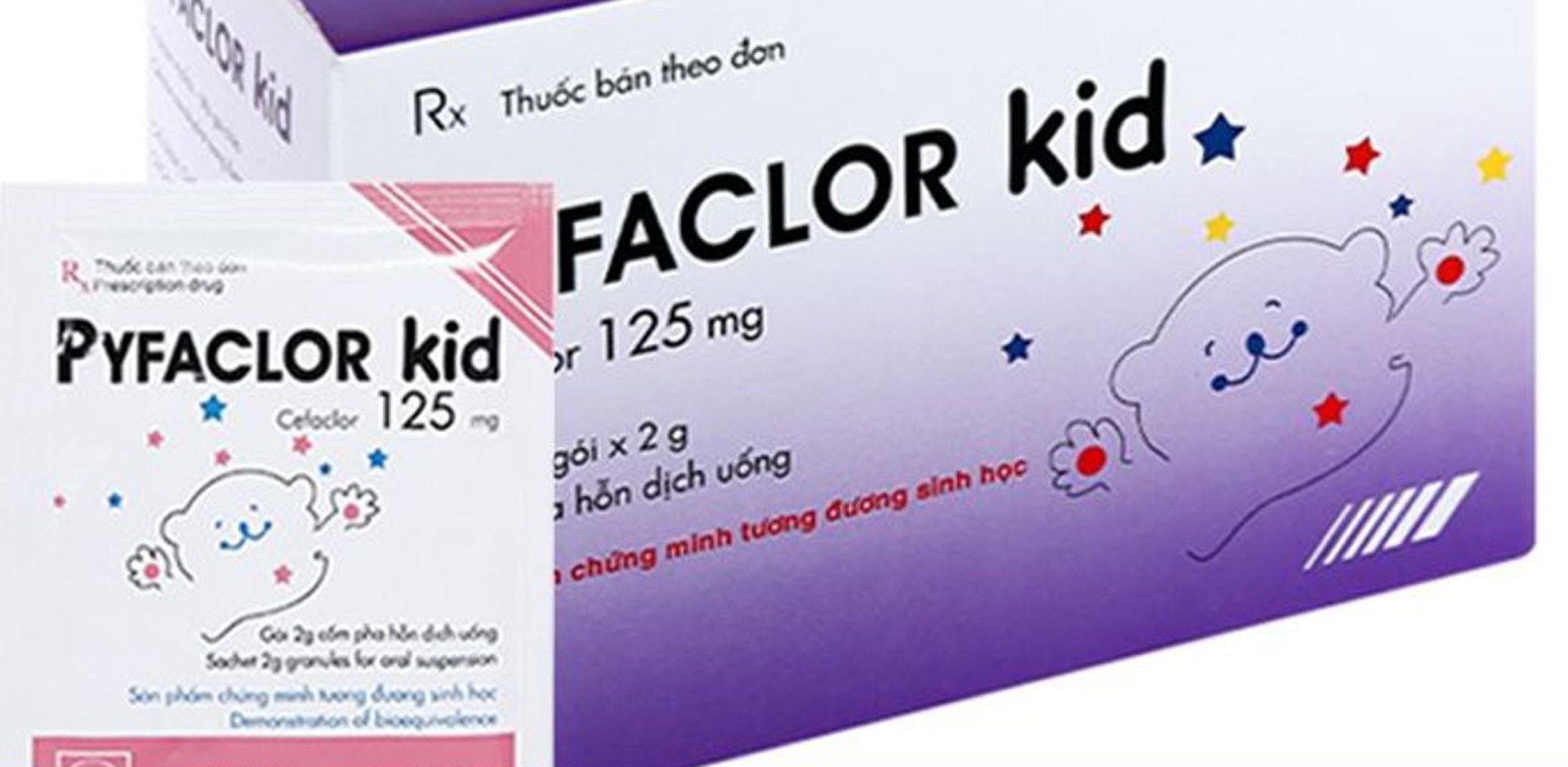
The Drug Administration of Vietnam has just issued a decision to recall nationwide the batch of Pyfaclor Kid (Cefaclor 125mg) oral suspension granules, registration number VD-26427-17, batch number 410923, production date September 13, 2023, expiry date September 13, 2026, manufactured by Pymepharco Pharmaceutical Joint Stock Company.
The Drug Administration requires Pymepharco Company to be responsible for stopping business, quarantining and notifying all wholesalers, retailers, hospitals, pharmacies and users who have received the product of the recall. The entire batch of drugs must be recalled and handled according to regulations within 15 days from the date of issuance of the decision. At the same time, the company must report the distribution situation, recall results and handling to the Drug Administration and the Department of Health of Quang Tri and Dak Lak within the prescribed time limit.
Wholesalers, retailers, hospitals and pharmacies must immediately stop selling, distributing and using this batch of medicine; at the same time, coordinate to recall and return it to the supplier. Patients are advised to immediately stop using products from the recalled batch.
The Department of Health of provinces and cities is responsible for publishing information, inspecting and supervising the implementation, and strictly handling related violations.
Source: https://baolaocai.vn/thu-hoi-toan-quoc-lo-thuoc-com-pha-hon-dich-uong-pyfaclor-kid-post883334.html



![[Photo] Panorama of the 2025 Community Action Awards Final Round](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/15/1763206932975_chi-7868-jpg.webp)

![[Photo] General Secretary To Lam receives Vice President of Luxshare-ICT Group (China)](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/15/1763211137119_a1-bnd-7809-8939-jpg.webp)
![[Photo] Prime Minister Pham Minh Chinh meets with representatives of outstanding teachers](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/11/15/1763215934276_dsc-0578-jpg.webp)






























































































Comment (0)