The Drug Administration of Vietnam ( Ministry of Health ) has just announced the suspension of circulation and nationwide recall of the cosmetic batch BIS UP ICE CARE TOOTHPASTE - 100g tube (Lot number: OJ4B; Manufacturing date: October 20, 2020; Expiry date: October 19, 2023) due to not meeting quality standards.
 |
Bis up ice care Toothpaste is recalled. |
The product label shows the GP CBMP number 139040/20/CBMP-QLD; manufacturer Green Wonil Co., Ltd; imported and distributed by Imexco Vietnam Trading Joint Stock Company, address: Lot 02 - 04 Hoang Mai Handicraft Cluster, Hoang Van Thu Ward, Hoang Mai District, Hanoi City.
Accordingly, in a document sent to the Department of Health of provinces and centrally run cities and Imexco Vietnam Trading Joint Stock Company, the Drug Administration of Vietnam said that the Ho Chi Minh City Center for Drug, Cosmetic and Food Testing had tested this batch of cosmetics and found that the test samples did not meet the quality standards for the limit of microorganisms in cosmetics as prescribed.
The Drug Administration of Vietnam requests the Department of Health of provinces and centrally-run cities to notify cosmetic businesses and users in the area to immediately stop selling and using the above batch of BIS UP ICE CARE TOOTHPASTE - 100g tube and return the product to the supplier.
At the same time, recall the above-mentioned batch of violating products; inspect and supervise units implementing this notice; handle violating units according to current regulations.
For Imexco Vietnam Trading Joint Stock Company, the Drug Administration requires sending a recall notice to the distributors and users of the above batch of BIS UP ICE CARE TOOTHPASTE - 100g tube. Receive returned products from business establishments, recall and destroy all batches of products that do not meet regulations, as well as send a recall report of the above batch of products to the Drug Administration before June 10.
The Drug Administration also requested the Hanoi Department of Health to inspect Imexco Vietnam Trading Joint Stock Company in complying with the provisions of the law on importing and trading cosmetics. Supervise the company to recall the batch of BIS UP ICE CARE TOOTHPASTE - 100g tube that does not meet the regulations. Handle and punish violations according to current regulations and report the results to the Drug Administration before June 30.
(According to mekongasean.vn)
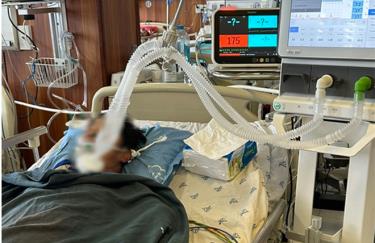
The 43-year-old male patient was admitted to the emergency room in a state of fatigue, difficulty breathing, shock, and arrhythmia.

The Ministry of Health has just issued regulations on the implementation of smoking-free areas.

These diet products do not promote long-term body fat loss and are instead linked to a higher risk of diabetes and heart disease.

Mr. Nguyen Trong Khoa - Deputy Director of the Department of Medical Examination and Treatment Management said that the Ministry of Health has requested medical examination and treatment facilities to prepare plans and strategies to be ready to return to work in case of an outbreak.
Source link





![[Photo] Prime Minister concludes trip to attend G20 Summit in South Africa](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F24%2F1763944494358_vna-potal-thu-tuong-ket-thuc-chuyen-tham-du-hoi-nghi-thuong-dinh-g20-tai-nam-phi-8428321-4810-jpg.webp&w=3840&q=75)




































































































Comment (0)