Pneumococcal vaccine 20 (PCV20) produced by Pfizer Pharmaceutical Group (USA) helps effectively prevent 20 strains of pneumococcal bacteria: 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F. These are the strains that cause many invasive pneumococcal diseases (septic pneumonia, meningitis, sepsis) and non-invasive pneumococcal diseases (pneumonia, otitis media, sinusitis, etc.).
Pneumococcal vaccine 20 is for adults aged 18 and over, and will continue to be used for children in the near future. The vaccine is currently used in nearly 50 countries around the world , including developed countries such as the US, UK, Germany, France, Australia, etc., showing clear effectiveness in reducing the incidence and mortality of pneumococcal disease in adults.
Pneumococcal vaccine 20 is produced at the world's leading modern vaccine factory in Belgium using advanced technology, proven to create a high level of immune response and long-term protection for users, while reducing the rate of healthy people carrying the virus, thereby reducing the possibility of disease spread in the community.
Dr. Bach Thi Chinh, Medical Director of VNVC Vaccination System, said that pneumococcus is the leading cause of disease burden and death in children and adults. The World Health Organization (WHO) estimates that about 1.6 million children and adults die from pneumococcus globally each year. It is also the cause of lower respiratory tract infections (LRTI) such as bronchiolitis, bronchitis, pneumonia, etc. with the highest mortality rate.
Pneumococcus has more than 100 serotypes that cause diverse and complex diseases, and antibiotic resistance is increasingly serious. Research and application of pneumococcal vaccines that can protect, cover more strains, reduce resistance rates, and abuse of antibiotics in treatment is a common and urgent trend in global medicine.
Therefore, scientists at many research institutes and vaccine manufacturers around the world have been constantly working to research and develop new pneumococcal vaccines with increasingly high effectiveness and safety. This is especially important to better protect public health, especially in the context of increasing respiratory diseases worldwide.
According to Dr. Bach Thi Chinh, the research and production technology of new vaccines by pharmaceutical companies has made great progress, thanks to which we have continuously had more types of vaccines, which can protect against more strains of pneumococcus, with higher protection efficiency and safety. Thanks to that, people have more flexible vaccination options, suitable for their age, health status, immune status and epidemiological characteristics in each locality.
The addition of the new generation PCV20 pneumococcal vaccine to the VNVC Vaccination System has helped expand the scope of protection and disease prevention effectiveness, especially modern technology helps reduce booster doses, thereby reducing vaccination costs and medical examination and treatment costs caused by pneumococcus.
Regarding the vaccination schedule, Dr. Bach Thi Chinh said: “Currently, the 20-valent pneumococcal vaccine is approved for adults aged 18 and older with a single vaccination schedule. People at high risk of pneumococcal diseases will be advised by their doctor on an appropriate vaccination schedule. For people who have been previously vaccinated with pneumococcal vaccines that cover fewer or more strains but require a booster shot, the doctor will consider prescribing a sequential or combination vaccination schedule to achieve the most optimal protection.”
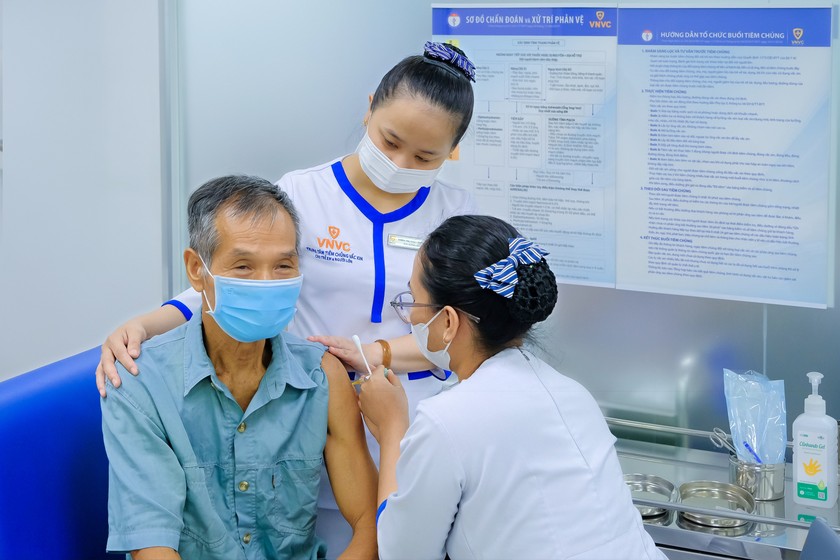
Adults receive pneumococcal vaccine 20 at VNVC Vaccination System. Photo: Moc Thao |
Thus, in just 2 weeks, VNVC has consecutively introduced two new pneumococcal vaccines into the community, affirming VNVC's pioneering role in quickly updating global medical advances, opening up opportunities for Vietnamese children and adults to have full access to the world's leading new, effective and safe vaccines.
In addition to cooperating with the world's leading vaccine and pharmaceutical companies to bring to Vietnam many new types of vaccines, new generation vaccines, increasing opportunities for Vietnamese people to access vaccines on par with those in developed countries, VNVC also took a strategic step of building the VNVC Vaccine and Biological Products Factory in Long An province. The factory has an initial investment of about 2,000 billion VND, is designed with modern technology with the world's leading technology, diverse and flexible in the ability to produce many different types of vaccines, aiming to proactively supply vaccines to people in the country, aiming to participate in the global vaccine supply chain, ready to meet the demand for vaccines to prevent epidemics in the future.
VNVC has also reached cooperation agreements with many major partners in vaccine research and production in the world such as Sanofi pharmaceutical company (France), Pfizer pharmaceutical company (USA), Gamaleya National Research Center for Epidemiology and Microbiology, Binnopharm Pharmaceutical Group of Russia,... to exchange research and transfer modern vaccine production technology.
Loc Tan
Source: https://baophapluat.vn/vnvc-trien-khai-tiem-vaccine-moi-phe-cau-20-cong-nghe-hien-dai-tai-viet-nam-post549710.html































































































Comment (0)