The Drug Administration of Vietnam has requested local health departments and provinces and cities across the country to urgently recall, inspect and trace the origin of the fake drug batch to ensure safety for users.
Regarding the counterfeit drug Clorocid TW3-Tablets (Cloramphenicol 250mg), registration number VD-25305-16, manufactured by Central Pharmaceutical Joint Stock Company 3 and packaged in plastic bottles of 400 tablets, the Department has repeatedly warned that products manufactured after September 15, 2019 are counterfeit drugs. The reason is that Central Pharmaceutical Joint Stock Company 3 stopped producing this drug after that time.
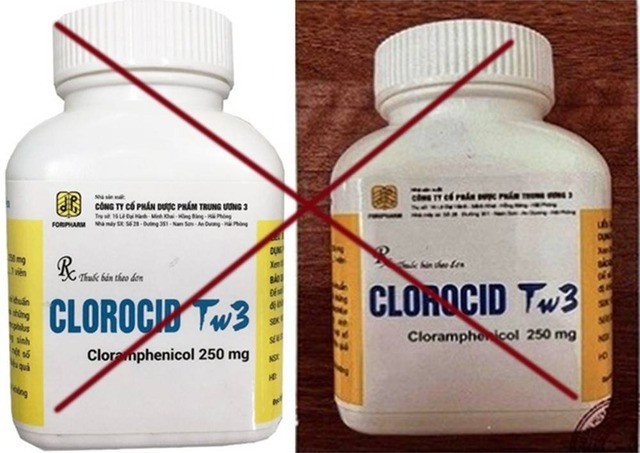
The Drug Administration of Vietnam continues to receive Official Dispatch No. 1739/KNTMPTP-KHTCKT dated November 28, 2025, accompanied by Test Certificate No. 2026/KNT-25 of the Hanoi Center for Drug, Cosmetic and Food Testing, reporting on the product sample with information printed on the label of Clorocid TW3 Tablets (Cloramphenicol 250mg), registration number VD-25305-16, batch number 3333, production date 06/06/2024, expiry date 06/06/2027, packaged in a plastic bottle of 400 tablets; place of manufacture: Central Pharmaceutical Joint Stock Company 3. The drug sample was taken by the Center for Drug, Cosmetic and Food Testing at Duc Hien Pharmacy, address: No. 58, Le Trong Tan Street, Ha Dong Ward, Hanoi City. The drug sample did not have a qualitative reaction of Chloramphenicol, it was a fake drug.
To ensure user safety, the Drug Administration of Vietnam requests the Hanoi Department of Health to urgently inspect compliance with the law in pharmaceutical business at Duc Hien Pharmacy, where counterfeit Clorocid TW3 products are sold. The Department must coordinate with relevant agencies to urgently recall the product, report to Steering Committee 389, and coordinate with the police, market management and functional agencies to trace the origin of the counterfeit drug batch. The results of the inspection and handling must be reported to the Drug Administration of Vietnam before December 6, 2025.
For the Department of Health of provinces and cities, the Department requests to widely notify drug businesses and users and people not to buy, sell or use fake Clorocid TW3 products.
Localities need to strengthen supervision, inspection, and coordination with authorities to verify the origin of invoices and documents of products if they appear in the area; promptly detect and handle the production and trade of counterfeit drugs.
The Drug Administration of Vietnam also requested units to thoroughly grasp and seriously implement the direction of the Ministry of Health in Official Dispatch No. 7724/BYT-QLD dated November 6, 2025 on stepping up the prevention of smuggling, trade fraud, and counterfeit goods in the medical field.
In addition, the media is requested to increase reporting, warn people to stop using fake Clorocid TW3 and notify authorities when detecting suspicious products.
Source: https://baohatinh.vn/bo-y-te-canh-bao-thuoc-clorocid-tw3-bi-lam-gia-post300676.html



![[Photo] National Assembly Chairman Tran Thanh Man attends the VinFuture 2025 Award Ceremony](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F05%2F1764951162416_2628509768338816493-6995-jpg.webp&w=3840&q=75)
![[Photo] 60th Anniversary of the Founding of the Vietnam Association of Photographic Artists](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F05%2F1764935864512_a1-bnd-0841-9740-jpg.webp&w=3840&q=75)




































































































Comment (0)