On September 16th, a representative from the Department of Pharmaceuticals stated that Novartis Vietnam had received four complaints from users regarding suspected counterfeit eye drops, including Tobrex, Maxitrol, and TobraDex. Verification confirmed that the 5ml Tobrex batch (VEE90A) was counterfeit. Three other batches, including 5ml Tobrex (batch VEE98C), 5ml Maxitrol (batch VFD09A), and 5ml TobraDex (batch VHN07A), were also suspected of being counterfeit. These products were found circulating outside the company's official distribution network. The representative from the Department of Pharmaceuticals stated that these products are widely available on the market.
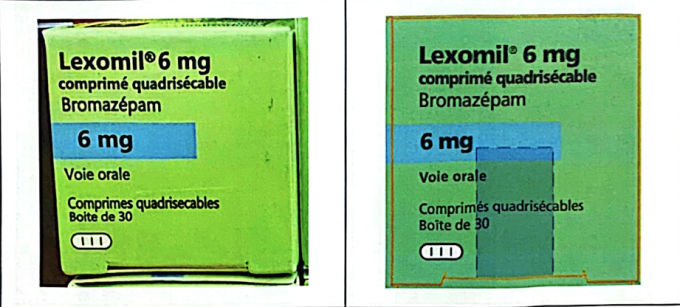
The second case involves the high-dose sleeping pill Lexomil 6 mg, owned by Cheplapharm Arzneimottel GmbH. Ho Chi Minh City police seized batch number F3193F01, with an expiration date of December 2027, which the manufacturer confirmed was counterfeit. This batch was originally produced for distribution in the French market and was not imported into Vietnam. Currently, Lexomil 6 mg does not have a registration number for domestic circulation; therefore, any trading or use of this product is illegal.
Finally, there's Aclasta (containing zoledronic acid), used to treat osteoporosis. A pharmacy in Kien Giang reported a product with a manufacturing date of August 2024 and an expiration date of July 2027. The company registering the product confirmed that this batch was not imported through official channels and that the packaging had irregularities. Specifically, genuine Aclasta manufactured after May 2024 has replaced the Novartis logo with the Sandoz logo. Therefore, products manufactured after this date but still bearing the old logo are suspected of being counterfeit.
In light of this situation, the Drug Administration requested local Departments of Health to notify hospitals, pharmacies, and the public not to trade or use these drugs. The assigned units were instructed to urgently inspect, monitor, and trace the origin of the infringing products.
The regulatory agency advises people to carefully check the information on the packaging and compare it with the data on licensed drugs on the Drug Administration's portal. If any suspicious signs are detected, people should immediately report them to the authorities for timely action.
Source: https://baohatinh.vn/bo-y-te-canh-bao-3-loai-thuoc-bi-nghi-lam-gia-post295756.html


![[Photo] Prime Minister Pham Minh Chinh holds a phone call with the CEO of Russia's Rosatom Corporation.](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F11%2F1765464552365_dsc-5295-jpg.webp&w=3840&q=75)












































































































Comment (0)