The Drug Administration Department requests that wholesale and retail establishments, as well as chain pharmacy businesses, cease trading, supplying, and distributing the recalled batch of drugs mentioned above; and that medical examination and treatment facilities and drug users cease prescribing, selling, dispensing, and using the recalled batch of drugs.
According to the Ministry of Health , in 2024, among the samples tested for quality control, the rate of substandard drugs was 0.45%, the rate of counterfeit drugs was less than 0.1%, and no counterfeit drugs were detected at medical examination and treatment facilities.
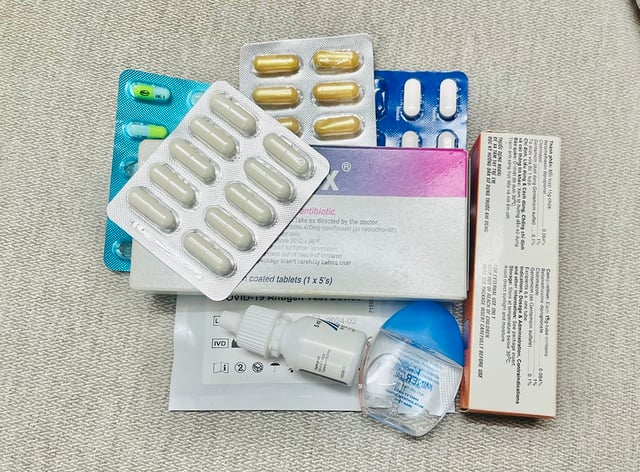
Each year, nearly 38,000 samples of circulating drugs in the country are sampled and tested.
PHOTO: NAM SON
Every year, the health sector collects and tests nearly 38,000 samples of drugs circulating in the market; the results show that the rate of substandard drugs has decreased sharply from over 10% in the early 1990s to less than 2% in recent years.
Regarding the assurance of drug and medical supply, the Ministry of Health stated that from July 1, 2025, hospitals with autonomous operating expenses (group 2 autonomy) and autonomous investment and operating expenses (group 1 autonomy) will be allowed to independently decide on the procurement of drugs and medical equipment from hospital revenue, health insurance funds, and other non-state budget sources without necessarily having to go through the bidding process as stipulated in the Law on Bidding.
Current regulations have simplified procedures, increased transparency, and improved the efficiency of contractor selection. These solutions help ensure a basic and sufficient supply of medicines and medical equipment for treatment and healthcare.
Source: https://thanhnien.vn/khong-phat-hien-thuoc-gia-tai-cac-co-so-kham-chua-benh-185251003180930536.htm


































![[Photo] Prime Minister Pham Minh Chinh holds a phone call with the CEO of Russia's Rosatom Corporation.](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F11%2F1765464552365_dsc-5295-jpg.webp&w=3840&q=75)









































































Comment (0)