
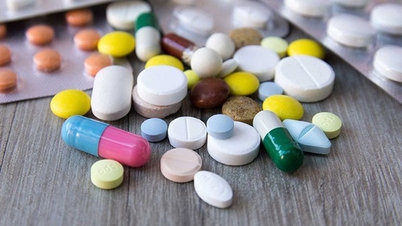
Gammaphil facial cleanser is distributed by GAMA Company.
Nationwide recall of the infringing product batch and revocation of the product declaration certificate.
This product is manufactured by GAMMA Pharmaceutical and Cosmetics Manufacturing Co., Ltd. (formerly GAMMA Private Enterprise for the Production of Chemical and Cosmetics), with its office located at 18 Nguyen Hau Street, Tan Thanh Ward, Tan Phu District, Ho Chi Minh City. The manufacturing facility is located at Group 1, Phu Hiep Hamlet, Phu Hoa Dong Commune, Cu Chi District, Ho Chi Minh City.
The recalled product batch has batch number GMPA010524, production date 2-5-2024, expiry date 2-5-2027, and product declaration form acceptance number 000669/21/CBMP-HCM.
According to test report No. 25L-020MP dated June 17th from the Center for Testing Drugs, Cosmetics, and Food - Yen Bai Provincial Department of Health, the tested product sample contained two substances, Methylparaben and Propylparaben (preservatives commonly used in cosmetics), but these were not declared in the published product formula.
Therefore, the Drug Administration of Vietnam requests the suspension of circulation and urgent recall of the aforementioned product batch nationwide. At the same time, it requires all businesses and users of cosmetics to immediately cease selling or using this batch of facial cleanser and return it to the supplier.
The Drug Administration Department requires GAMMA Company to send recall notices to all distributors and users of the product batch; receive back the products from business establishments; and proceed with the recall and destruction of the entire batch of infringing products. Simultaneously, a report on the recall and destruction must be submitted to the Drug Administration Department before August 2, 2025.
The Ho Chi Minh City Department of Health is responsible for supervising the recall and destruction process by the enterprise and handling any violations according to regulations.
In addition, the procedure for revoking the product declaration registration number 000669/21/CBMP-HCM must be carried out in accordance with regulations.
The Drug Administration of Vietnam has requested the Ho Chi Minh City Department of Health to conduct a comprehensive inspection of GAMMA Company's cosmetic production and business activities, ensuring compliance with current legal regulations on cosmetics.
Many products have been recalled.
Previously, on May 27th, the Drug Administration also issued a decision to suspend circulation, order the recall and destruction of the entire batch of Adaphil Gentle Skin Cleanser - 125ml bottle with batch number ADSX010324 distributed by GAMMA Company.
The reason for the recall is that test results showed the product sample contained two preservatives, Methylparaben and Propylparaben, which were not listed in the product declaration approved by the Drug Administration.
In October 2024, the company was also ordered by the Drug Administration to suspend the circulation, recall, and destroy two batches of Cerina products under the GAMMA brand because they contained 2-phenoxyethanol, an ingredient not declared in the cosmetic product's declared formula.
Experts recommend discontinuing the use of recalled products and contacting the place of purchase for instructions on how to return them. Consumers can report the recall to their local health department if they find the product still being sold after the recall order.
Source: https://tuoitre.vn/lai-thu-hoi-toan-quoc-lo-sua-rua-mat-cua-cong-ty-gamma-do-chua-chat-khong-cong-bo-20250711150932746.htm



![[Photo] Closing Ceremony of the 10th Session of the 15th National Assembly](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F11%2F1765448959967_image-1437-jpg.webp&w=3840&q=75)



![[Photo] Prime Minister Pham Minh Chinh holds a phone call with the CEO of Russia's Rosatom Corporation.](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F11%2F1765464552365_dsc-5295-jpg.webp&w=3840&q=75)















































![[OFFICIAL] MISA GROUP ANNOUNCES ITS PIONEERING BRAND POSITIONING IN BUILDING AGENTIC AI FOR BUSINESSES, HOUSEHOLDS, AND THE GOVERNMENT](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/12/11/1765444754256_agentic-ai_postfb-scaled.png)


















































Comment (0)