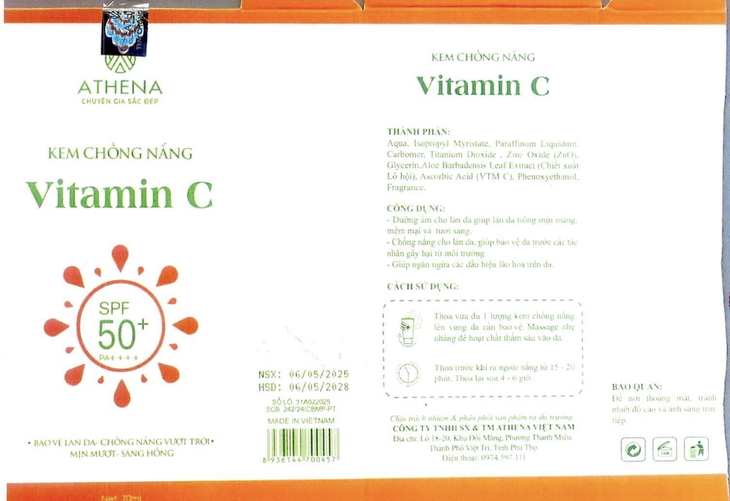
Athena's vitamin C sunscreen product was recalled - Photo: Department of Drug Administration
Sunscreen found to be "counterfeit"
The Drug Administration of Vietnam has just issued a decision to suspend circulation and recall nationwide two sunscreen products with signs of counterfeiting sun protection factor (SPF):
Specifically, Vitamin C sunscreen, cosmetic declaration receipt number 242/24/CBMP-PT issued on September 5, 2024. The product is distributed by Athena Vietnam Production and Trading Company Limited (address: Lot 18-20 Mang Hill Area, Thanh Mieu Ward, Viet Tri City, Phu Tho ).
Sun Cream sunscreen, cosmetic declaration receipt number 39/22/CBMP-PT issued on September 9, 2022. Product belongs to Lovis Cosmetics Company Limited (address: No. 64, Quang Trung Ward, Uong Bi City, Quang Ninh ).
According to the appraisal results at the Institute of Criminal Science of the Ministry of Public Security , the actual SPF index of both products was only below 70% compared to the declared on the label, seriously violating the regulations on labeling and advertising, and was determined to be counterfeit goods according to the conclusion of the Economic Police Department - Phu Tho Provincial Police.
The Drug Administration requires these units to send recall notices to distribution facilities, receive back the products and submit recall reports before August 7. The Phu Tho Department of Health and assigned provinces and cities coordinate to inspect, monitor and strictly handle organizations and individuals who violate the regulations.

Sun Cream sunscreen recalled - Photo: Department of Drug Administration
Vitamin E cream but no vitamin E
Also according to the Drug Administration, based on the test results from the Center for Drug, Cosmetic and Food Testing - Hanoi Department of Health, the product Vitamin E Mosturising Cream Enriched with Sunflowers Oil, with the label "Product of Thailand", was found to not contain vitamin E as declared, and did not meet the prescribed microbial limits.
The product has batch number 56, production date 1-1-2024, expiry date 1-1-2028, and was sampled and tested at two pharmacies in Hanoi: An An Pharmacy (153 TDP 14, Kien Hung Ward, Ha Dong District) and Thao Vy Pharmacy (122 Nguyen Van Giap Street, Cau Dien Ward, Nam Tu Liem District).
Notably, this product's entire label is printed in a foreign language, without a Vietnamese sub-label, without the unit responsible for bringing it to market, and has not been granted a receipt number for the imported cosmetic declaration form.
With the above serious violations, the Drug Administration requested to recall and destroy all products nationwide to ensure safety for users.
At the same time, local Health Departments are required to urgently notify cosmetic businesses and traders to immediately stop using and circulating this product, coordinate in tracing the origin and handling violations in accordance with regulations.
People are advised not to use cosmetic products of unknown origin, without information about the responsible unit, without Vietnamese sub-labels or showing signs of fake effects.
Using poor quality cosmetics not only wastes money but also poses many potential health risks, especially skin irritation, infection, long-term damage and even destruction of the skin's natural protective layer.
Source: https://tuoitre.vn/thu-hoi-kem-chong-nang-duoc-xac-dinh-la-hang-gia-kem-duong-vitamin-e-nhung-khong-co-vitamin-e-20250717163358278.htm


![[Photo] Cat Ba - Green island paradise](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F04%2F1764821844074_ndo_br_1-dcbthienduongxanh638-jpg.webp&w=3840&q=75)





































































































Comment (0)