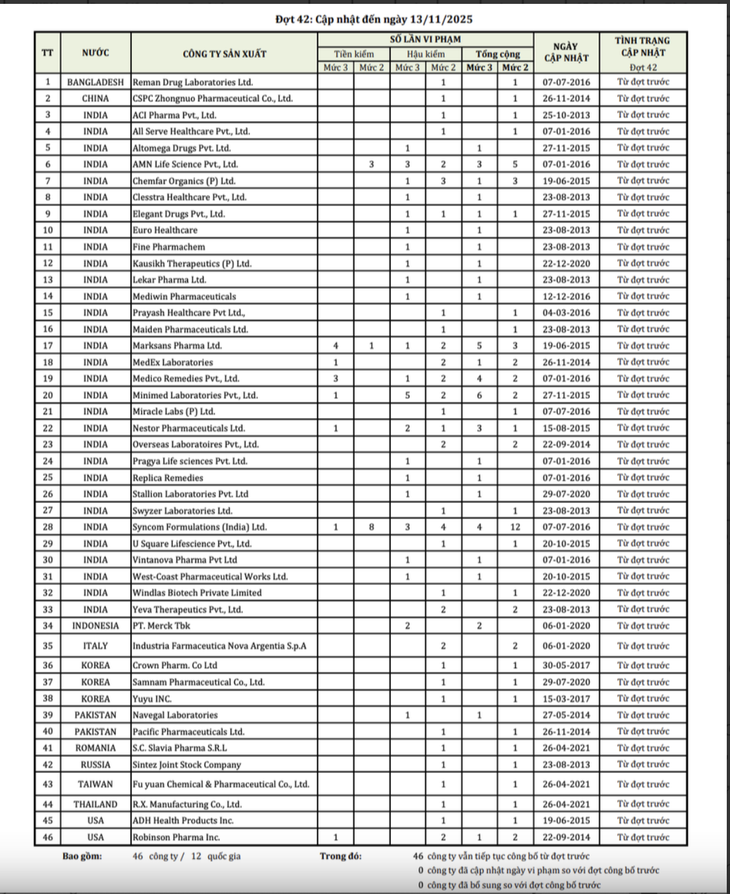
List of 46 foreign pharmaceutical companies with drugs violating quality updated to November 13, 2025 - Photo: Department of Drug Administration.
All 46 companies from 12 countries were cases of multiple recurrences, with the highest number of companies recurring up to 12 times.
The Drug Administration of Vietnam has just sent a document to the health departments of provinces and cities and drug importing companies to implement the regulations of the Ministry of Health on announcing, updating and removing names from the list of manufacturing facilities with drugs violating quality.
Accordingly, based on the results of monitoring the quality of drugs in circulation, reviewing facilities with violating drugs and foreign drug manufacturing facilities that are eligible to be removed from the list of those required to take samples for quality testing of 100% of imported drug batches, the Drug Administration Department announces the list of foreign companies with violating drugs.
Accordingly, in this list, Indian enterprises continue to be the country with the largest number of violating enterprises. A series of companies that appeared from 2013 - 2015 to date are still kept in the pre-inspection list.
In addition to the large group from India, many businesses from Bangladesh, China, Indonesia, South Korea, Pakistan, the US, Italy and Romania also continue to be under special surveillance.
Bangladesh's Reman Drug Laboratories, China's CSPC Zhongnuo, Indonesia's PT. Merck Tbk, and South Korea's Crown Pharm and Yuyu Inc. were all found guilty in earlier batches, but have yet to meet the requirements for the pre-inspection to be lifted.
In this list, there are 2 American companies such as ADH Health Products and Robinson Pharma, which have both pre-inspection and post-inspection quality violations.
Companies on this list must conduct quality testing sampling for 100% of imported drug batches (pre-inspection).
In addition to the list of violations, the Drug Administration also said that 98 companies from 16 countries were removed from the monitoring list after completing the pre-inspection period and no new violations occurred.
The Drug Administration of Vietnam requests the Department of Health of provinces, cities, and health sectors to direct drug management, inspection, and testing units under the department to conduct inspections and supervise compliance with regulations on quality inspection of imported drugs in circulation in the management area, and handle organizations/individuals who violate according to current regulations.
Source: https://tuoitre.vn/danh-sach-46-cong-ty-duoc-nuoc-ngoai-co-thuoc-vi-pham-chat-luong-20251203160328166.htm

































![[Photo] Parade to celebrate the 50th anniversary of Laos' National Day](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F02%2F1764691918289_ndo_br_0-jpg.webp&w=3840&q=75)









































































Comment (0)