In Official Dispatch 4261, the Drug Administration requested the Health Departments of provinces, cities and sectors to notify drug trading and using establishments about the characteristics and signs of counterfeit products as follows:
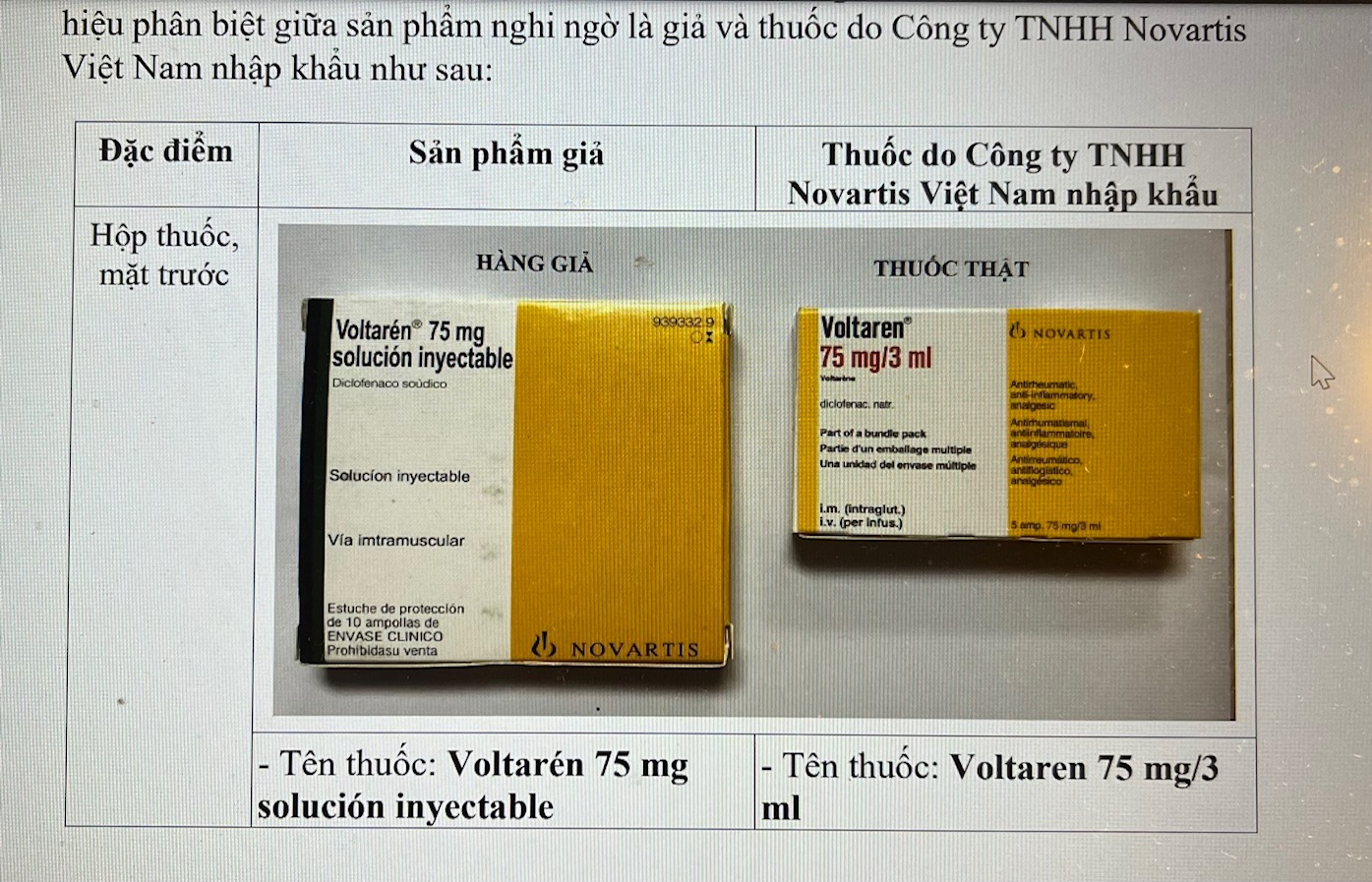
Drug name: Voltarén 75 mg solución inyectable. Batch number: 81111. Expiration date: 12.2023.
Manufacturer printed on the packaging: Novartis Farmacécutica, SA Gran Via de les Corrta Catalenes, 764 08013 Barcelona.
 |
Distinguishing fake Voltaren medicine |
Drug Administration |
The language on the box and syringe is only Spanish. There is no Vietnamese information printed or labeled on the packaging.
Box size: 12 cm wide; 10.5 cm long. The drug does not have a circulation registration number in Vietnam; has a barcode.
The Drug Administration of Vietnam recommends that health departments and sectors coordinate with media agencies to inform drug trading and using establishments and people not to trade or use Voltarén 75 mg solución inyectable products with the above-mentioned identifying signs.
Coordinate with relevant authorities to inspect drug trading establishments in the area; verify information and trace the origin of the above-mentioned products, promptly detect and prevent the production, trade and use of fake Voltarén 75 mg/3 ml. Report the results of inspection, handling of violations and the origin of the fake drug batch to the Department of Drug Administration.
According to the Drug Administration of Vietnam, the drug sample Voltaren 75 mg/3 ml in circulation is imported and supplied by Novartis Vietnam Company Limited with registration number: VN-20041-16 and manufactured by Lek Pharmaceuticals dd (Slovenia).
According to information from some treatment units, Voltaren 75mg has anti-inflammatory pain relief effects, indicated for the treatment of: osteoarthritis, ankylosing spondylitis, spondylitis, spinal pain syndrome, acute gout...
Source: https://thanhnien.vn/cuc-quan-ly-duoc-thong-bao-ve-thuoc-dieu-tri-dau-xuong-khop-voltaren-75-mg-gia-1851462631.htm































![[Photo] Worshiping the Tuyet Son statue - a nearly 400-year-old treasure at Keo Pagoda](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F02%2F1764679323086_ndo_br_tempimageomw0hi-4884-jpg.webp&w=3840&q=75)
![[Photo] Parade to celebrate the 50th anniversary of Laos' National Day](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F12%2F02%2F1764691918289_ndo_br_0-jpg.webp&w=3840&q=75)








































































Comment (0)